Activated R-ras, Rac1, PI 3-kinase and PKCepsilon can each restore cell spreading inhibited by isolated integrin beta1 cytoplasmic domains
- PMID: 11134082
- PMCID: PMC2150687
- DOI: 10.1083/jcb.151.7.1549
Activated R-ras, Rac1, PI 3-kinase and PKCepsilon can each restore cell spreading inhibited by isolated integrin beta1 cytoplasmic domains
Abstract
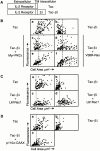
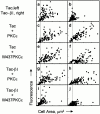
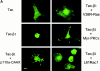
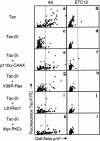
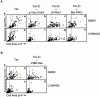
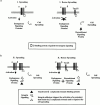
Attachment of many cell types to extracellular matrix proteins triggers cell spreading, a process that strengthens cell adhesion and is a prerequisite for many adhesion-dependent processes including cell migration, survival, and proliferation. Cell spreading requires integrins with intact beta cytoplasmic domains, presumably to connect integrins with the actin cytoskeleton and to activate signaling pathways that promote cell spreading. Several signaling proteins are known to regulate cell spreading, including R-Ras, PI 3-kinase, PKCepsilon and Rac1; however, it is not known whether they do so through a mechanism involving integrin beta cytoplasmic domains. To study the mechanisms whereby cell spreading is regulated by integrin beta cytoplasmic domains, we inhibited cell spreading on collagen I or fibrinogen by expressing tac-beta1, a dominant-negative inhibitor of integrin function, and examined whether cell spreading could be restored by the coexpression of either V38R-Ras, p110alpha-CAAX, myr-PKCepsilon, or L61Rac1. Each of these activated signaling proteins was able to restore cell spreading as assayed by an increase in the area of cells expressing tac-beta1. R-Ras and Rac1 rescued cell spreading in a GTP-dependent manner, whereas PKCstraightepsilon required an intact kinase domain. Importantly, each of these signaling proteins required intact beta cytoplasmic domains on the integrins mediating adhesion in order to restore cell spreading. In addition, the rescue of cell spreading by V38R-Ras was inhibited by LY294002, suggesting that PI 3-kinase activity is required for V38R-Ras to restore cell spreading. In contrast, L61Rac1 and myr-PKCstraightepsilon each increased cell spreading independent of PI 3-kinase activity. Additionally, the dominant-negative mutant of Rac1, N17Rac1, abrogated cell spreading and inhibited the ability of p110alpha-CAAX and myr-PKCstraightepsilon to increase cell spreading. These studies suggest that R-Ras, PI 3-kinase, Rac1 and PKCepsilon require the function of integrin beta cytoplasmic domains to regulate cell spreading and that Rac1 is downstream of PI 3-kinase and PKCepsilon in a pathway involving integrin beta cytoplasmic domain function in cell spreading.
Figures




References
-
- Burridge K., Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 1996;12:463–519. - PubMed
-
- Chen Y.-P., O'Toole T.E., Shipley T., Forsyth J., LaFlamme S.E., Yamada K.M., Shattil S.J., Ginsberg M.H. “Inside-out” signal transduction inhibited by isolated integrin cytoplasmic domains. J. Biol. Chem. 1994;269:18307–18310. - PubMed
-
- Chou M.M., Hou W., Johnson J., Graham L.K., Lee M.H., Chen C.-S., Newton A.C., Schaffhausen B.S., Toker A. Regulation of protein kinase C ζ by PI 3-kinase and PDK-1. Curr. Biol. 1998;8:1069–1077. - PubMed
-
- Chun J.-S., Ha M.-J., Jacobson B.S. Differential translocation of protein kinase C ε during HeLa cell adhesion to a gelatin substratum. J. Biol. Chem. 1996;271:13008–13012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

