Loss of calpain 3 proteolytic activity leads to muscular dystrophy and to apoptosis-associated IkappaBalpha/nuclear factor kappaB pathway perturbation in mice
- PMID: 11134085
- PMCID: PMC2150676
- DOI: 10.1083/jcb.151.7.1583
Loss of calpain 3 proteolytic activity leads to muscular dystrophy and to apoptosis-associated IkappaBalpha/nuclear factor kappaB pathway perturbation in mice
Abstract
Calpain 3 is known as the skeletal muscle-specific member of the calpains, a family of intracellular nonlysosomal cysteine proteases. It was previously shown that defects in the human calpain 3 gene are responsible for limb girdle muscular dystrophy type 2A (LGMD2A), an inherited disease affecting predominantly the proximal limb muscles. To better understand the function of calpain 3 and the pathophysiological mechanisms of LGMD2A and also to develop an adequate model for therapy research, we generated capn3-deficient mice by gene targeting. capn3-deficient mice are fully fertile and viable. Allele transmission in intercross progeny demonstrated a statistically significant departure from Mendel's law. capn3-deficient mice show a mild progressive muscular dystrophy that affects a specific group of muscles. The age of appearance of myopathic features varies with the genetic background, suggesting the involvement of modifier genes. Affected muscles manifest a similar apoptosis-associated perturbation of the IkappaBalpha/nuclear factor kappaB pathway as seen in LGMD2A patients. In addition, Evans blue staining of muscle fibers reveals that the pathological process due to calpain 3 deficiency is associated with membrane alterations.
Figures
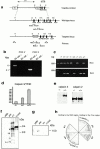
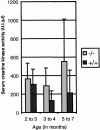
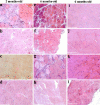

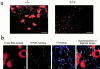
References
-
- Baghdiguian S., Martin M., Richard I., Pons F., Astier C., Bourg N., Hay R.T., Chemaly R., Halaby G., Loiselet J. Calpain 3 deficiency is associated with myonuclear apoptosis and profound perturbation of the IκBa/NF-kB pathway in limb-girdle muscular dystrophy type 2A. Nat. Med. 1999;5:503–511. - PubMed
-
- Baldwin A.S., Jr. The NFκB and IκB proteinsnew discoveries and insights. Annu. Rev. Immunol. 1996;14:649–681. - PubMed
-
- Bönnemann C.G., Modi R., Noguchi S., Mizuno Y., Yoshida M., Gussoni E., McNally E.M., Duggan D.J., Angelini C., Hoffman E.P., Ozawa E., Kunkel L.M. β-Sarcoglycan (A3b) mutations cause autosomal recessive muscular dystrophy with loss of the sarcoglycan complex. Nat. Genet. 1995;11:266–273. - PubMed
-
- Dear T.N., Boehm T. Diverse mRNA expression patterns of the mouse calpain genes Capn5, Capn6 and Capn11 during development. Mech. Dev. 1999;89:201–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

