Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice
- PMID: 11134178
- PMCID: PMC198546
- DOI: 10.1172/JCI10564
Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice
Abstract
It has been suggested that increased collagenase-3 (MMP-13) activity plays a pivotal role in the pathogenesis of osteoarthritis (OA). We have used tetracycline-regulated transcription in conjunction with a cartilage-specific promoter to target a constitutively active human MMP-13 to the hyaline cartilages and joints of transgenic mice. Postnatal expression of this transgene resulted in pathological changes in articular cartilage of the mouse joints similar to those observed in human OA. These included characteristic erosion of the articular cartilage associated with loss of proteoglycan and excessive cleavage of type II collagen by collagenase, as well as synovial hyperplasia. These results demonstrate that excessive MMP-13 activity can result in articular cartilage degradation and joint pathology of the kind observed in OA, suggesting that excessive activity of this proteinase can lead to this disease.
Figures

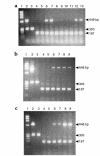

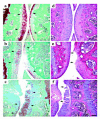


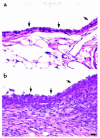
Comment in
-
Petulant cellular acts: destroying the ECM rather than creating it.J Clin Invest. 2001 Jan;107(1):31-2. doi: 10.1172/JCI11892. J Clin Invest. 2001. PMID: 11134176 Free PMC article. Review. No abstract available.
References
-
- Poole, A.R., Alini, M., and Hollander, A. 1995. Cellular biology of cartilage degradation. In Mechanisms and models in rheumatoid arthritis. B. Henderson, R Pettifer, and J. Edwards, editors. Academic Press. New York, New York, USA. 163–204.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

