Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response
- PMID: 11134185
- PMCID: PMC198545
- DOI: 10.1172/JCI10501
Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response
Abstract
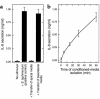
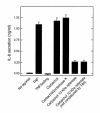
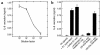
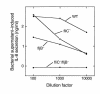
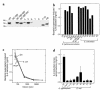
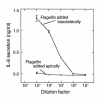
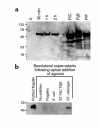
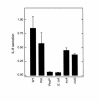
This study investigated whether soluble paracrine factors mediated Salmonella-induced IL-8 expression in polarized model intestinal epithelia. We found that the basolateral media of model epithelia that had been apically infected with Salmonella typhimurium for a short period (10 minutes) could activate IL-8 secretion in virgin model epithelia, demonstrating that a proinflammatory factor (PIF) was indeed present. Initial characterization found that PIF was a heat-stable protein with a molecular mass of about 50 kDa that acts on the basolateral, but not apical, surface of model intestinal epithelia to elicit IL-8 secretion. PIF was not present in the media of model epithelia stimulated with other inducers of IL-8 secretion (TNF-alpha or carbachol) but was present in S. typhimurium supernatants, indicating PIF is of bacterial origin. PIF was purified from bacterial culture supernatants by anion/cation exchange chromatography and SDS-PAGE and found by using microsequencing to be the protein flagellin. In support of this finding, flagellin-deficient S. typhimurium mutants did not secrete detectable levels of PIF (i.e., a bioactivity that induced IL-8 secretion when placed basolaterally on model epithelia). Furthermore, viable flagellin-deficient mutant organisms (fliC/fljB and flhD) failed to elicit IL-8 secretion when added apically to model intestinal epithelia. These findings indicate that translocation of flagellin across epithelia, subsequent to apical epithelial-S. typhimurium interaction, is likely a major means of activating a mucosal inflammatory response.
Figures


Comment in
-
Breaching the mucosal barrier by stealth: an emerging pathogenic mechanism for enteroadherent bacterial pathogens.J Clin Invest. 2001 Jan;107(1):27-30. doi: 10.1172/JCI11792. J Clin Invest. 2001. PMID: 11134175 Free PMC article. Review. No abstract available.
References
-
- Madara JL. Review article: pathobiology of neutrophil interactions with intestinal epithelia. Aliment Pharmacol Ther. 1997;11(Suppl. 3):57–62. - PubMed
-
- McCormick BA, Parkos CA, Colgan SP, Carnes DK, Madara JL. Apical secretion of a pathogen-elicited epithelial chemoattractant (PEEC) activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160:455–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

