Organization of immature human immunodeficiency virus type 1
- PMID: 11134289
- PMCID: PMC113972
- DOI: 10.1128/JVI.75.2.759-771.2001
Organization of immature human immunodeficiency virus type 1
Abstract
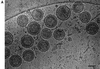
Immature retrovirus particles contain radially arranged Gag polyproteins in which the N termini lie at the membrane and the C termini extend toward the particle's center. We related image features to the polyprotein domain structure by combining mutagenesis with cryoelectron microscopy and image analysis. The matrix (MA) domain appears as a thin layer tightly associated with the inner face of the viral membrane, separated from the capsid (CA) layer by a low-density region corresponding to its C terminus. Deletion of the entire p6 domain has no effect on the width or spacing of the density layers, suggesting that p6 is not ordered in immature human immunodeficiency virus type 1 (HIV-1). In vitro assembly of a recombinant Gag polyprotein containing only capsid (CA) and nucleocapsid (NC) domains results in the formation of nonenveloped spherical particles which display two layers with density matching that of the CA-NC portion of immature HIV-1 Gag particles. Authentic, immature HIV-1 displays additional surface features and an increased density between the lipid bilayers which reflect the presence of gp41. The other internal features match those of virus-like particles.
Figures















References
-
- Coffin J M, Hughes S H, Varmus H E. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

