Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread
- PMID: 11134295
- PMCID: PMC113978
- DOI: 10.1128/JVI.75.2.821-833.2001
Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread
Abstract
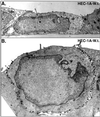
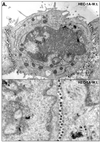
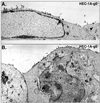
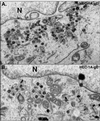
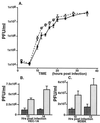
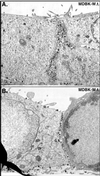
Alphaherpesviruses spread rapidly through dermal tissues and within synaptically connected neuronal circuitry. Spread of virus particles in epithelial tissues involves movement across cell junctions. Herpes simplex virus (HSV), varicella-zoster virus (VZV), and pseudorabies virus (PRV) all utilize a complex of two glycoproteins, gE and gI, to move from cell to cell. HSV gE/gI appears to function primarily, if not exclusively, in polarized cells such as epithelial cells and neurons and not in nonpolarized cells or cells that form less extensive cell junctions. Here, we show that HSV particles are specifically sorted to cell junctions and few virions reach the apical surfaces of polarized epithelial cells. gE/gI participates in this sorting. Mutant HSV virions lacking gE or just the cytoplasmic domain of gE were rarely found at cell junctions; instead, they were found on apical surfaces and in cell culture fluids and accumulated in the cytoplasm. A component of the AP-1 clathrin adapter complexes, mu1B, that is involved in sorting of proteins to basolateral surfaces was involved in targeting of PRV particles to lateral surfaces. These results are related to recent observations that (i) HSV gE/gI localizes specifically to the trans-Golgi network (TGN) during early phases of infection but moves out to cell junctions at intermediate to late times (T. McMillan and D. C. Johnson, J. Virol., in press) and (ii) PRV gE/gI participates in envelopment of nucleocapsids into cytoplasmic membrane vesicles (A. R. Brack, B. G. Klupp, H. Granzow, R. Tirabassi, L. W. Enquist, and T. C. Mettenleiter, J. Virol. 74:4004-4016, 2000). Therefore, interactions between the cytoplasmic domains of gE/gI and the AP-1 cellular sorting machinery cause glycoprotein accumulation and envelopment into specific TGN compartments that are sorted to lateral cell surfaces. Delivery of virus particles to cell junctions would be expected to enhance virus spread and enable viruses to avoid host immune defenses.
Figures


Similar articles
-
Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans-Golgi network, a site of virus envelopment and sorting of virions to cell junctions.J Virol. 2001 Feb;75(4):1928-40. doi: 10.1128/JVI.75.4.1928-1940.2001. J Virol. 2001. PMID: 11160692 Free PMC article.
-
Herpes simplex virus gE/gI must accumulate in the trans-Golgi network at early times and then redistribute to cell junctions to promote cell-cell spread.J Virol. 2006 Apr;80(7):3167-79. doi: 10.1128/JVI.80.7.3167-3179.2006. J Virol. 2006. PMID: 16537585 Free PMC article.
-
Herpes simplex virus gE/gI expressed in epithelial cells interferes with cell-to-cell spread.J Virol. 2003 Feb;77(4):2686-95. doi: 10.1128/jvi.77.4.2686-2695.2003. J Virol. 2003. PMID: 12552008 Free PMC article.
-
The role of herpes simplex virus glycoproteins in the virus replication cycle.Acta Virol. 1998 Apr;42(2):103-18. Acta Virol. 1998. PMID: 9770079 Review.
-
Herpesviral Fc receptors and their relationship to the human Fc receptors.Immunol Res. 1992;11(3-4):226-38. doi: 10.1007/BF02919129. Immunol Res. 1992. PMID: 1337547 Review.
Cited by
-
CD2v Interacts with Adaptor Protein AP-1 during African Swine Fever Infection.PLoS One. 2015 Apr 27;10(4):e0123714. doi: 10.1371/journal.pone.0123714. eCollection 2015. PLoS One. 2015. PMID: 25915900 Free PMC article.
-
Characterization of the Herpes Simplex Virus (HSV) Tegument Proteins That Bind to gE/gI and US9, Which Promote Assembly of HSV and Transport into Neuronal Axons.J Virol. 2020 Nov 9;94(23):e01113-20. doi: 10.1128/JVI.01113-20. Print 2020 Nov 9. J Virol. 2020. PMID: 32938770 Free PMC article.
-
Replication of herpes simplex virus: egress of progeny virus at specialized cell membrane sites.J Virol. 2012 Jul;86(13):7084-97. doi: 10.1128/JVI.00463-12. Epub 2012 Apr 24. J Virol. 2012. PMID: 22532674 Free PMC article.
-
Function of glycoprotein E of herpes simplex virus requires coordinated assembly of three tegument proteins on its cytoplasmic tail.Proc Natl Acad Sci U S A. 2012 Nov 27;109(48):19798-803. doi: 10.1073/pnas.1212900109. Epub 2012 Nov 12. Proc Natl Acad Sci U S A. 2012. PMID: 23150560 Free PMC article.
-
Herpes simplex virus type 2 glycoprotein G is targeted by the sulfated oligo- and polysaccharide inhibitors of virus attachment to cells.J Virol. 2007 Dec;81(24):13424-34. doi: 10.1128/JVI.01528-07. Epub 2007 Oct 10. J Virol. 2007. PMID: 17928351 Free PMC article.
References
-
- Babic N, Klupp B, Brack A, Mettenleiter T C, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–284. - PubMed
-
- Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75:1245–1258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

