Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model
- PMID: 11134298
- PMCID: PMC113981
- DOI: 10.1128/JVI.75.2.850-856.2001
Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model
Abstract
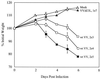
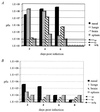
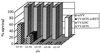
The vaccinia virus (VV) E3L gene is responsible for providing interferon (IFN) resistance and a broad host range to VV in cell culture. The E3L gene product contains two distinct domains. A conserved carboxy-terminal domain, which is required for the IFN resistance and broad host range of the virus, has been shown to bind double-stranded RNA (dsRNA) and inhibit the antiviral dsRNA-dependent protein kinase, PKR. The amino-terminal domain, while conserved among orthopoxviruses, is dispensable in cell culture. To study the role of E3L in whole-animal infections, WR strain VV recombinants either lacking E3L (VVDeltaE3L) or expressing an amino-terminal (VVE3LDelta83N) or carboxy-terminal (VVE3LDelta26C) truncation of E3L were constructed. Whereas wild-type VV had a 50% lethal dose of approximately 10(4) PFU after intranasal infection, and elicited severe weight loss and morbidity, VVDeltaE3L was apathogenic, leading to no death, weight loss, or morbidity. VVDeltaE3L was also apathogenic after intracranial injection. Although the amino-terminal domain of E3L is dispensable for infection of cells in culture, both the amino- and carboxy-terminal domains of E3L were required for full pathogenesis in intranasal infections. These results demonstrate that the entire E3L gene is required for pathogenesis in the mouse model.
Figures



References
-
- Bass B L, Hurst S R, Singer J D. Binding properties of newly identified Xenopus proteins containing dsRNA-binding motifs. Curr Biol. 1994;4:301–314. - PubMed
-
- Beattie E, Kauffman E B, Martinez H, Perkus M E, Jacobs B L, Paoletti E, Tartaglia J. Host-range restriction of vaccinia virus E3L-specific deletion mutants. Virus Genes. 1996;12:89–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

