Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells
- PMID: 11134315
- PMCID: PMC113998
- DOI: 10.1128/JVI.75.2.1013-1030.2001
Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells
Abstract
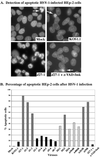
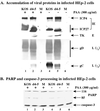
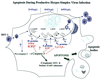
We previously reported that a recombinant ICP27-null virus stimulated, but did not prevent, apoptosis in human HEp-2 cells during infection (M. Aubert and J. A. Blaho, J. Virol. 73:2803-2813, 1999). In the present study, we used a panel of 15 recombinant ICP27 mutant viruses to determine which features of herpes simplex virus type 1 (HSV-1) replication are required for the apoptosis-inhibitory activity. Each virus was defined experimentally as either apoptotic, partially apoptotic, or nonapoptotic based on infected HEp-2 cell morphologies, percentages of infected cells with condensed chromatin, and patterns of specific cellular death factor processing. Viruses d27-1, d1-5, d1-2, M11, M15, M16, n504R, n406R, n263R, and n59R are apoptotic or partially apoptotic in HEp-2 cells and severely defective for growth in Vero cells. Viruses d2-3, d3-4, d4-5, d5-6, and d6-7 are nonapoptotic, demonstrating that ICP27 contains a large amino-terminal region, including its RGG box RNA binding domain, which is not essential for apoptosis prevention. Accumulations of viral TK, VP16, and gD but not gC, ICP22, or ICP4 proteins correlated with prevention of apoptosis during the replication of these viruses. Of the nonapoptotic viruses, d4-5 did not produce gC, indicating that accumulation of true late gene products is not necessary for the prevention process. Analyses of viral DNA synthesis in HEp-2 cells indicated that apoptosis prevention by HSV-1 requires that the infection proceeds to the stage in which viral DNA replication takes place. Infections performed in the presence of the drug phosphonoacetic acid confirmed that the process of viral DNA synthesis and the accumulation of true late (gamma(2)) proteins are not required for apoptosis prevention. Based on our results, we conclude that the accumulation of HSV-1 early (beta) and leaky-late (gamma(1)) proteins correlates with the prevention of apoptosis in infected HEp-2 cells.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

