Two-step nature of human T-cell leukemia virus type 1 replication in experimentally infected squirrel monkeys (Saimiri sciureus)
- PMID: 11134325
- PMCID: PMC114008
- DOI: 10.1128/JVI.75.2.1083-1089.2001
Two-step nature of human T-cell leukemia virus type 1 replication in experimentally infected squirrel monkeys (Saimiri sciureus)
Abstract
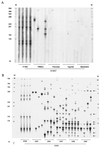
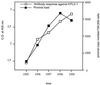
After experimental infection of squirrel monkeys (Saimiri sciureus) with human T-cell leukemia virus type 1 (HTLV-1)-infected cells, the virus is transcribed only transiently in circulating blood, spleen, and lymph nodes. Stable disappearance of viral expression occurs at 2 to 3 weeks after inoculation. This coincides with the development of the anti-HTLV-1 immune response and persistent detection of the provirus in peripheral blood mononuclear cells (PBMCs). In this study, the HTLV-1 replication pattern was analyzed over time in PBMCs and various organs from two HTLV-1-infected squirrel monkeys. Real-time quantitative PCR confirmed that PBMCs and lymphoid organs constitute the major reservoirs for HTLV-1. The PCR amplification of HTLV-1 flanking sequences from PBMCs evidenced a pattern of clonal expansion of infected cells identical to that observed in humans. Dissemination of the virus in body compartments appeared to result from cellular transport of the integrated provirus. The circulating proviral burden increased as a function of time in one animal studied over a period of 4 years. The high proviral loads observed in the last samples resulted from the accumulation of infected cells via the extensive proliferation of a restricted number of persistent clones on a background of polyclonally expanded HTLV-1-positive cells. Therefore, HTLV-1 primary infection in squirrel monkeys is a two-step process involving a transient phase of reverse transcription followed by persistent multiplication of infected cells. This suggests that the choice of the target for blocking HTLV-1 replication might depend on the stage of infection.
Figures
Similar articles
-
Lymphoid organs as a major reservoir for human T-cell leukemia virus type 1 in experimentally infected squirrel monkeys (Saimiri sciureus): provirus expression, persistence, and humoral and cellular immune responses.J Virol. 2000 May;74(10):4860-7. doi: 10.1128/jvi.74.10.4860-4867.2000. J Virol. 2000. PMID: 10775625 Free PMC article.
-
HTLV type 1 infection in squirrel monkeys (Saimiri sciureus): a promising animal model for HTLV type 1 human infection.AIDS Res Hum Retroviruses. 2000 Nov 1;16(16):1741-6. doi: 10.1089/08892220050193245. AIDS Res Hum Retroviruses. 2000. PMID: 11080820 Review.
-
HTLV-I infection in squirrel monkeys (Saïmiri sciureus) using autologous, homologous, or heterologous HTLV-I-transformed cell lines.Virology. 1997 May 12;231(2):258-66. doi: 10.1006/viro.1997.8528. Virology. 1997. PMID: 9168888
-
Quantification of HTLV-I proviral load in experimentally infected rabbits.Retrovirology. 2005 May 23;2:34. doi: 10.1186/1742-4690-2-34. Retrovirology. 2005. PMID: 15910683 Free PMC article.
-
High HTLV-1 Proviral Load Predates and Predicts HTLV-1-Associated Disease: Literature Review and the London Experience.Pathogens. 2024 Jul 1;13(7):553. doi: 10.3390/pathogens13070553. Pathogens. 2024. PMID: 39057780 Free PMC article. Review.
Cited by
-
HTLV-1 ORF-I Encoded Proteins and the Regulation of Host Immune Response: Viral Induced Dysregulation of Intracellular Signaling.J Immunol Res. 2015;2015:498054. doi: 10.1155/2015/498054. Epub 2015 Oct 18. J Immunol Res. 2015. PMID: 26557721 Free PMC article. Review.
-
Novel Treatments of Adult T Cell Leukemia Lymphoma.Front Microbiol. 2020 May 28;11:1062. doi: 10.3389/fmicb.2020.01062. eCollection 2020. Front Microbiol. 2020. PMID: 32547515 Free PMC article. Review.
-
Endemic Viruses of Squirrel Monkeys (Saimiri spp.).Comp Med. 2015 Jun;65(3):232-40. Comp Med. 2015. PMID: 26141448 Free PMC article. Review.
-
One-step, multiplex, real-time PCR assay with molecular beacon probes for simultaneous detection, differentiation, and quantification of human T-cell leukemia virus types 1, 2, and 3.J Clin Microbiol. 2009 Apr;47(4):1129-35. doi: 10.1128/JCM.02006-08. Epub 2009 Feb 11. J Clin Microbiol. 2009. PMID: 19213697 Free PMC article.
-
Peripheral lymphocytosis presenting as EBV/HTLV-1 co-infection adult T-cell leukemia.Hematol Transfus Cell Ther. 2022 Apr-Jun;44(2):279-283. doi: 10.1016/j.htct.2020.09.146. Epub 2020 Nov 21. Hematol Transfus Cell Ther. 2022. PMID: 33281112 Free PMC article. No abstract available.
References
-
- Agape P, Copin M C, Cavrois M, Panelatti G, Plumelle Y, Ossondo-Landeau M, Quist D, Grossat N, Gosselin B, Fenaux P, Wattel E. Implication of HTLV-I infection, strongyloidiasis, and P53 overexpression in the development, response to treatment, and evolution of non-Hodgkin's lymphomas in an endemic area (Martinique, French West Indies) J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:394–402. - PubMed
-
- Albrecht B, Collins N D, Newbound G C, Ratner L, Lairmore M D. Quantification of human T-cell lymphotropic virus type 1 proviral load by quantitative competitive polymerase chain reaction. J Virol Methods. 1998;75:123–140. - PubMed
-
- Cavrois M, Gessain A, Gout O, Wain-Hobson S, Wattel E. Common human T cell leukemia virus type 1 (HTLV-1) integration sites in cerebrospinal fluid and blood lymphocytes of patients with HTLV-1-associated myelopathy/tropical spastic paraparesis indicate that HTLV-1 crosses the blood-brain barrier via clonal HTLV-1-infected cells. J Infect Dis. 2000;182:1044–1050. - PubMed
-
- Cavrois M, Gessain A, Wain-Hobson S, Wattel E. Proliferation of HTLV-1 infected circulating cells in vivo in all asymptomatic carriers and patients with TSP/HAM. Oncogene. 1996;12:2419–2423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

