Two-step nature of human T-cell leukemia virus type 1 replication in experimentally infected squirrel monkeys (Saimiri sciureus)
- PMID: 11134325
- PMCID: PMC114008
- DOI: 10.1128/JVI.75.2.1083-1089.2001
Two-step nature of human T-cell leukemia virus type 1 replication in experimentally infected squirrel monkeys (Saimiri sciureus)
Abstract
After experimental infection of squirrel monkeys (Saimiri sciureus) with human T-cell leukemia virus type 1 (HTLV-1)-infected cells, the virus is transcribed only transiently in circulating blood, spleen, and lymph nodes. Stable disappearance of viral expression occurs at 2 to 3 weeks after inoculation. This coincides with the development of the anti-HTLV-1 immune response and persistent detection of the provirus in peripheral blood mononuclear cells (PBMCs). In this study, the HTLV-1 replication pattern was analyzed over time in PBMCs and various organs from two HTLV-1-infected squirrel monkeys. Real-time quantitative PCR confirmed that PBMCs and lymphoid organs constitute the major reservoirs for HTLV-1. The PCR amplification of HTLV-1 flanking sequences from PBMCs evidenced a pattern of clonal expansion of infected cells identical to that observed in humans. Dissemination of the virus in body compartments appeared to result from cellular transport of the integrated provirus. The circulating proviral burden increased as a function of time in one animal studied over a period of 4 years. The high proviral loads observed in the last samples resulted from the accumulation of infected cells via the extensive proliferation of a restricted number of persistent clones on a background of polyclonally expanded HTLV-1-positive cells. Therefore, HTLV-1 primary infection in squirrel monkeys is a two-step process involving a transient phase of reverse transcription followed by persistent multiplication of infected cells. This suggests that the choice of the target for blocking HTLV-1 replication might depend on the stage of infection.
Figures
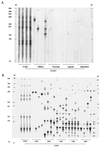
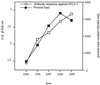
References
-
- Agape P, Copin M C, Cavrois M, Panelatti G, Plumelle Y, Ossondo-Landeau M, Quist D, Grossat N, Gosselin B, Fenaux P, Wattel E. Implication of HTLV-I infection, strongyloidiasis, and P53 overexpression in the development, response to treatment, and evolution of non-Hodgkin's lymphomas in an endemic area (Martinique, French West Indies) J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:394–402. - PubMed
-
- Albrecht B, Collins N D, Newbound G C, Ratner L, Lairmore M D. Quantification of human T-cell lymphotropic virus type 1 proviral load by quantitative competitive polymerase chain reaction. J Virol Methods. 1998;75:123–140. - PubMed
-
- Cavrois M, Gessain A, Gout O, Wain-Hobson S, Wattel E. Common human T cell leukemia virus type 1 (HTLV-1) integration sites in cerebrospinal fluid and blood lymphocytes of patients with HTLV-1-associated myelopathy/tropical spastic paraparesis indicate that HTLV-1 crosses the blood-brain barrier via clonal HTLV-1-infected cells. J Infect Dis. 2000;182:1044–1050. - PubMed
-
- Cavrois M, Gessain A, Wain-Hobson S, Wattel E. Proliferation of HTLV-1 infected circulating cells in vivo in all asymptomatic carriers and patients with TSP/HAM. Oncogene. 1996;12:2419–2423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

