Copper-modulated gene expression and senescence in the filamentous fungus Podospora anserina
- PMID: 11134328
- PMCID: PMC86578
- DOI: 10.1128/MCB.21.2.390-399.2001
Copper-modulated gene expression and senescence in the filamentous fungus Podospora anserina
Abstract
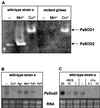
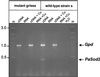
We have previously shown that the control of cellular copper homeostasis by the copper-modulated transcription factor GRISEA has an important impact on the phenotype and lifespan of Podospora anserina. Here we demonstrate that copper depletion leads to the induction of an alternative respiratory pathway and to an increase in lifespan. This response compensates mitochondrial dysfunctions via the expression of PaAox, a nuclear gene coding for an alternative oxidase. It resembles the retrograde response in Saccharomyces cerevisiae. In P. anserina, this pathway appears to be induced by specific impairments of the copper-dependent cytochrome c oxidase. It is not induced as the result of a general decline of mitochondrial functions during senescence. We cloned and characterized PaAox. Transcript levels are decreased when cellular copper, superoxide, and hydrogen peroxide levels are raised. Copper also controls transcript levels of PaSod2, the gene encoding the mitochondrial manganese superoxide dismutase (PaSOD2). PaSod2 is a target of transcription factor GRISEA. During the senescence of wild-type strain s, the activity of PaSOD2 decreases, whereas the activity of the cytoplasmic copper/zinc superoxide dismutase (PaSOD1) increases. Collectively, the data explain the postponed senescence of mutant grisea as a defined consequence of copper depletion, ultimately leading to a reduction of oxidative stress. Moreover, they suggest that during senescence of the wild-type strain, copper is released from mitochondria. The involved mechanism is unknown. However, it is striking that the permeability of mitochondrial membranes in animal systems changes during apoptosis and that mitochondrial proteins with an important impact on this type of cellular death are released.
Figures

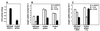


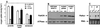


References
-
- Albury M S, Affourtit C, Moore A L. A highly conserved glutamate residue (Glu-270) is essential for plant alternative oxidase activity. J Biol Chem. 1998;273:30301–30305. - PubMed
-
- Amarvadi R, Glerum D M, Tzagoloff A. Isolation of a cDNA encoding the human homolog of COX17, a yeast gene essential for mitochondrial copper recruitment. Hum Genet. 1997;99:329–333. - PubMed
-
- Andersson M E, Nordlund P. A revised model of the active site of alternative oxidase. FEBS Lett. 1999;449:17–22. - PubMed
-
- Averbeck, N. B., C. Borghouts, A. Hamann, V. Specke, and H. D. Osiewacz. Molecular control of cellular copper homeostasis in filamentous fungi: increased expression of a metallothionein gene during aging of Podospora anserina. Mol. Gen. Genet., in press. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
