Irradiation promotes V(D)J joining and RAG-dependent neoplastic transformation in SCID T-cell precursors
- PMID: 11134329
- PMCID: PMC86582
- DOI: 10.1128/MCB.21.2.400-413.2001
Irradiation promotes V(D)J joining and RAG-dependent neoplastic transformation in SCID T-cell precursors
Abstract
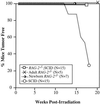
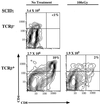
Defects in the nonhomologous end-joining (NHEJ) pathway of double-stranded DNA break repair severely impair V(D)J joining and selectively predispose mice to the development of lymphoid neoplasia. This connection was first noted in mice with the severe combined immune deficient (SCID) mutation in the DNA-dependent protein kinase (DNA-PK). SCID mice spontaneously develop thymic lymphoma with low incidence and long latency. However, we and others showed that low-dose irradiation of SCID mice dramatically increases the frequency and decreases the latency of thymic lymphomagenesis, but irradiation does not promote the development of other tumors. We have used this model to explore the mechanistic basis by which defects in NHEJ confer selective and profound susceptibility to lymphoid oncogenesis. Here, we show that radiation quantitatively and qualitatively improves V(D)J joining in SCID cells, in the absence of T-cell receptor-mediated cellular selection. Furthermore, we show that the lymphocyte-specific endonuclease encoded by the recombinase-activating genes (RAG-1 and RAG-2) is required for radiation-induced thymic lymphomagenesis in SCID mice. Collectively, these data suggest that irradiation induces a DNA-PK-independent NHEJ pathway that facilitates V(D)J joining, but also promotes oncogenic misjoining of RAG-1/2-induced breaks in SCID T-cell precursors.
Figures



Similar articles
-
Involvement of illegitimate V(D)J recombination or microhomology-mediated nonhomologous end-joining in the formation of intragenic deletions of the Notch1 gene in mouse thymic lymphomas.Cancer Res. 2004 Dec 15;64(24):8882-90. doi: 10.1158/0008-5472.CAN-03-1163. Cancer Res. 2004. PMID: 15604248
-
V(D)J recombination is not required for the development of lymphoma in p53-deficient mice.Cell Growth Differ. 1998 Feb;9(2):131-8. Cell Growth Differ. 1998. PMID: 9486849
-
p53 is required for both radiation-induced differentiation and rescue of V(D)J rearrangement in scid mouse thymocytes.Genes Dev. 1996 Mar 1;10(5):553-65. doi: 10.1101/gad.10.5.553. Genes Dev. 1996. PMID: 8598286
-
V(D)J recombination deficiencies.Adv Exp Med Biol. 2009;650:46-58. doi: 10.1007/978-1-4419-0296-2_4. Adv Exp Med Biol. 2009. PMID: 19731800 Review.
-
Cutting apart V(D)J recombination.Curr Opin Genet Dev. 1996 Apr;6(2):141-5. doi: 10.1016/s0959-437x(96)80042-6. Curr Opin Genet Dev. 1996. PMID: 8722168 Review.
Cited by
-
The RAG proteins in V(D)J recombination: more than just a nuclease.Nucleic Acids Res. 2001 Apr 1;29(7):1399-409. doi: 10.1093/nar/29.7.1399. Nucleic Acids Res. 2001. PMID: 11266539 Free PMC article. Review.
-
A Lamina-Associated Domain Border Governs Nuclear Lamina Interactions, Transcription, and Recombination of the Tcrb Locus.Cell Rep. 2018 Nov 13;25(7):1729-1740.e6. doi: 10.1016/j.celrep.2018.10.052. Cell Rep. 2018. PMID: 30428344 Free PMC article.
-
Spontaneous nonthymic tumors in SCID mice.Comp Med. 2011 Jun;61(3):227-34. Comp Med. 2011. PMID: 21819692 Free PMC article.
-
Molecular nature of radiation injury and DNA repair disorders associated with radiosensitivity.Int J Hematol. 2012 Mar;95(3):239-45. doi: 10.1007/s12185-012-1008-y. Epub 2012 Feb 18. Int J Hematol. 2012. PMID: 22351161 Review.
References
-
- Agrawal A, Eastman Q M, Schatz D G. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature. 1998;394:744–751. - PubMed
-
- Agrawal A, Schatz D G. RAG1 and RAG2 form a stable postcleavage synaptic complex with DNA containing signal ends in V(D)J recombination. Cell. 1997;89:43–53. - PubMed
-
- Anderson S J, Levin S D, Perlmutter R M. Protein tyrosine kinase p56lck controls allelic exclusion of T-cell receptor β-chain genes. Nature. 1993;365:552–554. - PubMed
-
- Aplan P D, Lombardi D P, Ginsberg A M, Cossman J, Bertness V L, Kirsch I R. Disruption of the human SCL locus by “illegitimate” V-(D)-J recombinase activity. Science. 1990;250:1426–1429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
