Oligomerization of DH domain is essential for Dbl-induced transformation
- PMID: 11134331
- PMCID: PMC86589
- DOI: 10.1128/MCB.21.2.425-437.2001
Oligomerization of DH domain is essential for Dbl-induced transformation
Abstract
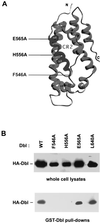
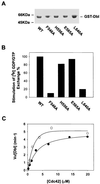
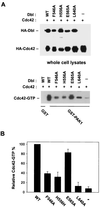
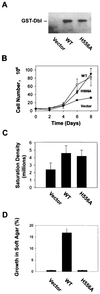
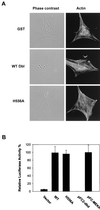
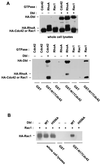
The dbl oncogene product (onco-Dbl) is the prototype member of a family of guanine nucleotide exchange factors (GEFs) for Rho GTPases. The Dbl homology (DH) domain of onco-Dbl is responsible for the GEF catalytic activity, and the DH domain, together with the immediately adjacent pleckstrin homology (PH) domain, constitutes the minimum module bearing transforming function. In the present study, we demonstrate that the onco-Dbl protein exists in oligomeric form in vitro and in cells. The oligomerization is mostly homophilic in nature and is mediated by the DH domain. Mutagenesis studies mapped the region involved in oligomerization to the conserved region 2 of the DH domain, which is located at the opposite side of the Rho GTPase interacting surface. Residue His556 of this region, in particular, is important for this activity, since the H556A mutant retained the GEF catalytic capability and the binding activity toward Cdc42 and RhoA in vitro but was deficient in oligomer formation. Consequently, the Rho GTPase activating potential of the H556A mutant was significantly reduced in cells. The focus-forming and anchorage-independent growth activities of onco-Dbl were completely abolished by the His556-to-Ala mutation, whereas the abilities to stimulate cell growth, activate Jun N-terminal kinase, and cause actin cytoskeletal changes were retained by the mutant. The ability of onco-Dbl to oligomerize allowed multiple Rho GTPases to be recruited to the same signaling complex, and such an ability is defective in the H556A mutant. Taken together, these results suggest that oligomerization of onco-Dbl through the DH domain is essential for cellular transformation by providing the means to generate a signaling complex that further augments and/or coordinates its Rho GTPase activating potential.
Figures




References
-
- Abe K, Whitehead I P, O'Bryan J P, Der C J. Involvement of NH2-terminal sequences in the negative regulation of Vav signaling and transforming activity. J Biol Chem. 1999;274:30410–30418. - PubMed
-
- Aghazadeh B, Zhu K, Kubiseski T J, Liu G A, Pawson T, Zheng Y, Rosen M K. Structure and mutagenesis of the Dbl homology domain. Nat Struct Biol. 1998;12:1098–1107. - PubMed
-
- Aghazadeh B, Lowry W E, Huang X-Y, Rosen M K. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–633. - PubMed
-
- Boriack-Sjodin P A, Margarit S M, Bar-Sagi D, Kuriyan J. The structural basis of the activation of Ras by Sos. Nature. 1998;394:337–343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
