Emergence of ocular dominance columns in cat visual cortex by 2 weeks of age
- PMID: 11135259
- PMCID: PMC2412906
- DOI: 10.1002/1096-9861(20010205)430:2<235::aid-cne1028>3.0.co;2-p
Emergence of ocular dominance columns in cat visual cortex by 2 weeks of age
Abstract
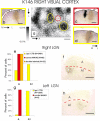
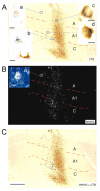
Previous anatomic studies of the geniculocortical projection showed that ocular dominance columns emerge by 3 weeks of age in cat visual cortex, but recent optical imaging experiments have revealed a pattern of physiologic eye dominance by the end of the second week of life. We used two methods to search for an anatomic correlate of this early functional ocular dominance pattern. First, retrograde labeling of lateral geniculate nucleus (LGN) inputs to areas of cortex preferentially activated by one eye showed that the geniculocortical projection was already partially segregated by eye at postnatal day 14 (P14). Second, transneuronal label of geniculocortical afferents in flattened sections of cortex after a tracer injection into one eye showed a periodic pattern at P14 but not at P7. In the classic model for the development of ocular dominance columns, initially overlapping geniculocortical afferents segregate by means of an activity-dependent competitive process. Our data are consistent with this model but suggest that ocular dominance column formation begins between P7 and P14, approximately a week earlier than previously believed. The functional and anatomic data also reveal an early developmental bias toward contralateral eye afferents. This initial developmental bias is not consistent with a strictly Hebbian model for geniculocortical afferent segregation. The emergence of ocular dominance columns before the onset of the critical period for visual deprivation also suggests that the mechanisms for ocular dominance column formation may be partially distinct from those mediating plasticity later in life.
Copyright 2001 Wiley-Liss, Inc.
Figures








References
-
- Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993a;260:1819–1821. - PubMed
-
- Basbaum AI, Menetrey D. Wheat germ agglutinin-apoHRP gold: a new retrograde tracer for light- and electron-microscopic single- and double-label studies. J Comp Neurol. 1986;261:306–318. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

