Cleavage of cyclin A at R70/R71 by the bacterial protease OmpT
- PMID: 11136238
- PMCID: PMC14615
- DOI: 10.1073/pnas.98.2.497
Cleavage of cyclin A at R70/R71 by the bacterial protease OmpT
Abstract
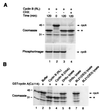
Previous work has shown that cyclin A can be cleaved at Arg-70/Arg-71 by a proteolytic activity present in an in vitro-coupled transcription/translation system by using rabbit reticulocyte lysate programmed by plasmid DNA encoding p27(KIP1), a cyclin-dependent kinase inhibitor, but not by plasmid DNAs encoding other cyclin-dependent kinases inhibitors. Here we report that cyclin A is also cleaved by translation product programmed by plasmid DNA encoding cyclin B. Several findings indicate that the cleavage activity in this assay is provided by the bacterial protease OmpT, which cofractionates with cyclin B and p27(KIP1) plasmid DNAs and is thus carried over into the coupled in vitro transcription/translation reactions. (i) Cleavage activity appeared even when transcription or translation of the cyclin B or p27(KIP1) was blocked. (ii) Activity resembling OmpT, a serine protease that cleaves between dibasic residues, routinely copurifies with p27(KIP1) and cyclin B plasmid DNAs. (iii) Both cyclin A cleavage activity and OmpT activity are heat stable, resistant to denaturation, and inhibited by Zn(2+), Cu(2+), or benzamidine. (iv) Cyclin A cleavage activity is detected when using lysates or DNAs prepared from Escherichia coli strains that contained OmpT but not with strains lacking OmpT. (v) Purified OmpT enzyme itself cleaves cyclin A at R70/R71. These data indicate that OmpT can be present in certain DNA preparations obtained by using standard plasmid purification protocols, and its presence can potentially affect the outcome and interpretation of studies carried out using in vitro-translated proteins.
Figures





Similar articles
-
The cyclin-dependent kinase inhibitor p27(Kip1) induces N-terminal proteolytic cleavage of cyclin A.Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15374-81. doi: 10.1073/pnas.95.26.15374. Proc Natl Acad Sci U S A. 1998. PMID: 9860976 Free PMC article.
-
BCR/ABL regulates expression of the cyclin-dependent kinase inhibitor p27Kip1 through the phosphatidylinositol 3-Kinase/AKT pathway.J Biol Chem. 2000 Dec 15;275(50):39223-30. doi: 10.1074/jbc.M007291200. J Biol Chem. 2000. PMID: 11010972
-
Utilization of Escherichia coli outer-membrane endoprotease OmpT variants as processing enzymes for production of peptides from designer fusion proteins.Appl Environ Microbiol. 2004 Jan;70(1):76-86. doi: 10.1128/AEM.70.1.76-86.2004. Appl Environ Microbiol. 2004. PMID: 14711628 Free PMC article.
-
Low levels of cyclin D and nonfunctional Rb protein affect cdk6 association with cyclin-dependent kinase inhibitor p27(Kip1).Biochem Biophys Res Commun. 2001 Jun 1;284(1):106-11. doi: 10.1006/bbrc.2001.4947. Biochem Biophys Res Commun. 2001. PMID: 11374878
-
Antimicrobial Peptide Conformation as a Structural Determinant of Omptin Protease Specificity.J Bacteriol. 2015 Nov;197(22):3583-91. doi: 10.1128/JB.00469-15. Epub 2015 Sep 8. J Bacteriol. 2015. PMID: 26350132 Free PMC article.
Cited by
-
Improving health from the inside: Use of engineered intestinal microorganisms as in situ cytokine delivery system.Bioengineered. 2013 May-Jun;4(3):172-9. doi: 10.4161/bioe.22646. Epub 2012 Oct 30. Bioengineered. 2013. PMID: 23111320 Free PMC article.
-
BCL-W is a regulator of microtubule inhibitor-induced mitotic cell death.Oncotarget. 2016 Jun 21;7(25):38718-38730. doi: 10.18632/oncotarget.9586. Oncotarget. 2016. PMID: 27231850 Free PMC article.
-
Substrate specificity of the Escherichia coli outer membrane protease OmpP.J Bacteriol. 2007 Jan;189(2):522-30. doi: 10.1128/JB.01493-06. Epub 2006 Nov 3. J Bacteriol. 2007. PMID: 17085556 Free PMC article.
-
The kinetics of p53 activation versus cyclin E accumulation underlies the relationship between the spindle-assembly checkpoint and the postmitotic checkpoint.J Biol Chem. 2008 Jun 6;283(23):15716-23. doi: 10.1074/jbc.M800629200. Epub 2008 Apr 9. J Biol Chem. 2008. PMID: 18400748 Free PMC article.
-
Enhanced fluorescent properties of an OmpT site deleted mutant of green fluorescent protein.Microb Cell Fact. 2010 Apr 29;9:26. doi: 10.1186/1475-2859-9-26. Microb Cell Fact. 2010. PMID: 20429908 Free PMC article.
References
-
- Poon R Y C. In: Encyclopedia of Cancer. Bertino J R, editor. San Diego: Academic; 1996. pp. 246–255.
-
- Morgan D O. Annu Rev Cell Dev Biol. 1997;13:261–291. - PubMed
-
- Glotzer M, Murray A W, Kirschner M W. Nature (London) 1991;349:132–138. - PubMed
-
- Hershko A, Ganoth D, Pehrson J, Palazzo R E, Cohen L H. J Biol Chem. 1991;266:16376–16379. - PubMed
-
- Townsley F, Ruderman J V. Trends Cell Biol. 1998;8:238–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

