Engineering of a functional human NADH-dependent cytochrome P450 system
- PMID: 11136248
- PMCID: PMC14548
- DOI: 10.1073/pnas.98.1.81
Engineering of a functional human NADH-dependent cytochrome P450 system
Abstract
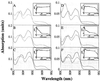
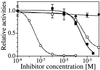
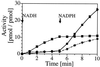
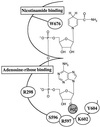
A functional human NADH-dependent cytochrome P450 system has been developed by altering the cofactor preference of human NADPH cytochrome P450 reductase (CPR), the redox partner for P450s. This has been achieved by a single amino acid change of the conserved aromatic amino acid Trp-676, which covers the re-side of the FAD isoalloxazine ring in the nicotinamide-binding site. Of the mutations made, the substitution of Trp-676 with alanine (W676A) resulted in a functional NADH-dependent enzyme, which catalyzed the reduction of cytochrome c and ferricyanide as well as facilitated the metabolism of 7-ethoxyresorufin by CYP1A2. Kinetic analysis measuring cytochrome c activity revealed that the NADH-dependent k(cat) of W676A is equivalent (90%) to the NADPH-dependent k(cat) of the wild-type enzyme, with W676A having an approximately 1,000-fold higher specificity for NADH. The apparent K(M)(NADPH) and K(M)(NADH) values of W676A are 80- and 150-fold decreased, respectively. In accordance with structural data, which show a bipartite binding mode of NADPH, substitution of Trp-676 does not affect 2'-AMP binding as seen by the inhibition of both wild-type CPR and the W676A mutant. Furthermore, NADPH was a potent inhibitor of the W676A NADH-dependent cytochrome c reduction and CYP1A2 activity. Overall, the results show that Trp-676 of human CPR plays a major role in cofactor discrimination, and substitution of this conserved aromatic residue with alanine results in an efficient NADH-dependent cytochrome P450 system.
Figures
References
-
- Backes W L. In: Cytochrome P-450. Schenkman J B, Greim H, editors. New York: Springer; 1993. pp. 35–59.
-
- Strobel H W, Hodgson A V, Shen S. In: Cytochrome P450: Structure, Mechanism and Biochemistry. Ortiz de Montellano P R, editor. New York: Plenum; 1995. p. 225. -244.
-
- Vermillon J L, Coon M J. J Biol Chem. 1978;253:2694–2704. - PubMed
-
- Walton M I, Wolf C R, Workman P. Biochem Pharmacol. 1992;44:251–259. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

