Multiplex allele-specific target amplification based on PCR suppression
- PMID: 11136256
- PMCID: PMC14569
- DOI: 10.1073/pnas.98.1.206
Multiplex allele-specific target amplification based on PCR suppression
Abstract
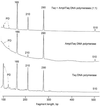
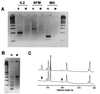
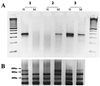
We have developed a strategy for multiplex PCR based on PCR suppression. PCR suppression allows DNA target amplification with only one sequence-specific primer per target and a second primer that is common for all targets. Therefore, an n-plex PCR would require only n + 1 primers. We have demonstrated uniform, efficient amplification of targeted sequences in 14-plex PCR. The high specificity of suppression PCR also provides multiplexed amplification with allele specificity. Multiplexed PCR was used to develop assays for genotyping DNA samples from cystic fibrosis-affected individuals. The new approach greatly simplifies primer design, significantly increases the PCR multiplexing level, and decreases the overall primer cost. In addition, this assay is more readily amenable to automation and is therefore suitable for high-throughput genetic diagnostics.
Figures



References
-
- Edwards M C, Gibbs R A. PCR Methods Appl. 1994;3:S65–S75. - PubMed
-
- Hacia J G, Sun B, Hunt N, Edgemon K, Mosbrook D, Robbins C, Fodor S P, Tagle D A, Collins F S. Genome Res. 1998;8:1245–1258. - PubMed
-
- Stuven T, Griese E U, Kroemer H K, Eichelbaum M, Zanger U M. Pharmacogenetics. 1996;6:417–421. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

