Population PK and PK/PD modelling of microencapsulated octreotide acetate in healthy subjects
- PMID: 11136293
- PMCID: PMC2015017
- DOI: 10.1046/j.1365-2125.2000.00297.x
Population PK and PK/PD modelling of microencapsulated octreotide acetate in healthy subjects
Abstract
Aims: To develop a population model that can describe the pharmacokinetic profile of microencapsulated octreotide acetate in healthy cholecystectomized subjects. To investigate the correlation between serum IGF-1 and octreotide concentration.
Methods: A population pharmacokinetic analysis was performed on octreotide data obtained following a single dose of 30 mg microencapsulated octreotide acetate intramuscularly. The relationship between serum IGF-1 concentration and octreotide concentration was effectively described by a population pharmacokinetic/pharmacodynamic model.
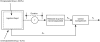
Results: The pharmacokinetic profile of octreotide was characterized by an initial peak of octreotide followed by a sustained-release of drug. Plateau concentration were sustained up to day 70, and gradually declined to below the detection limit by day 112. A one-compartment linear model was constructed which consisted of two absorption processes, characterized by KIR and KSR, rate constants for immediate-release and sustained-release, respectively, with first-order elimination (Ke; 1.05 h-1). The surface, unencapsulated drug was immediately absorbed into the central compartment with first-order absorption (KIR; 0.0312 h-1), while the microencapsulated drug was first released in a zero-order fashion into a depot before being absorbed into the central compartment with first-order absorption (KSR; 0.00469 h-1) during a period of tau (1680 h). Body weight and gender were important covariates for the apparent volume of distribution. The type of formulation was an important covariate for KIR but had no effect on KSR. An inhibitory Emax population pharmacokinetic/pharmacodynamic model could adequately describe the relationship between IGF-1 (expressed as percent baseline) and octreotide concentration. Baseline IGF-1 concentration was found to be a significant covariate for the baseline effect (E0). A relationship between GH concentration and octreotide concentration was not established.
Conclusions: The pharmacokinetic profile of microencapsulated octreotide acetate was effectively described by the derived population model. The relationship between IGF-1 and drug concentration could be used to guide optimization of therapeutic octreotide dosage regimens.
Figures
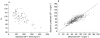
 ) (b; r2 = 0.72; 469 observations/63 treatments). — line of identity.
) (b; r2 = 0.72; 469 observations/63 treatments). — line of identity.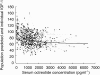
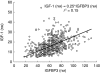
 serum IGF-1 concentrations (expressed as percent baseline) vs simultaneously measured serum octreotide concentrations (469 observations/63 treatments).
serum IGF-1 concentrations (expressed as percent baseline) vs simultaneously measured serum octreotide concentrations (469 observations/63 treatments).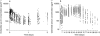
 (study 1);
(study 1);  B (study 2);
B (study 2);  C (study 2);
C (study 2);  B (study 2).
B (study 2).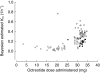
 A (study 1);
A (study 1);  C (study 2);
C (study 2);  B (studies 1 and 2).
B (studies 1 and 2).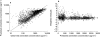
 C (study 2);
C (study 2);  B (study 2);
B (study 2);  A (study 1);
A (study 1);  (study 1);
(study 1); line of identity; Weighted residuals vs population predicted octreotide concentrations based on population parameter estimates (Model 5) (b; 3927 observations/110 treatments).
line of identity; Weighted residuals vs population predicted octreotide concentrations based on population parameter estimates (Model 5) (b; 3927 observations/110 treatments).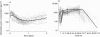
 ) for a typical female subject with body weight of 78 kg and receiving 30 mg C intramuscularly (a: 0—2 days; b: 2—112 days; 3927 observations).
) for a typical female subject with body weight of 78 kg and receiving 30 mg C intramuscularly (a: 0—2 days; b: 2—112 days; 3927 observations).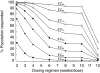
References
-
- Katz D, Erstad BL. Octreotide, a new somatostatin analogue. Clin Pharm. 1989;8:255–273. - PubMed
-
- Chanson P, Timsit J, Harris AG. Clinical pharmacokinetics of octreotide. Therapeutic applications in patients with pituitary tumors. Clin Pharmacokin. 1993;25:375–391. - PubMed
-
- Lamberts W. Somatostatin analogs: their role in the treatment of growth hormone hypersecretion and excessive body growth. Growth Regul. 1991;1:3–10. - PubMed
-
- Grass P, Marbach P, Bruns C, Lancranjan I. Sandostatin LAR (microencapsulated octreotide acetate) in acromegaly: pharmacokinetic and pharmacodynamic relationships. Metabolism. 1996;45:27–30. - PubMed
-
- Lancranjan I, Bruns P, Grass P. Sandostatin® LAR®: a promising therapeutic tool in the management of acromegalic patients. Metabolism. 1996;45(Suppl 1):67–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

