Benzydamine N-oxidation as an index reaction reflecting FMO activity in human liver microsomes and impact of FMO3 polymorphisms on enzyme activity
- PMID: 11136294
- PMCID: PMC2015007
- DOI: 10.1046/j.1365-2125.2000.00296.x
Benzydamine N-oxidation as an index reaction reflecting FMO activity in human liver microsomes and impact of FMO3 polymorphisms on enzyme activity
Abstract
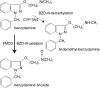
Aims: The role of flavin containing monooxygenases (FMO) on the disposition of many drugs has been insufficiently explored. In vitro and in vivo tests are required to study FMO activity in humans. Benzydamine (BZD) N-oxidation was evaluated as an index reaction for FMO as was the impact of genetic polymorphisms of FMO3 on activity.
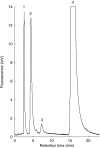
Methods: BZD was incubated with human liver microsomes (HLM) and recombinant enzymes. Human liver samples were genotyped using PCR-RFLP.
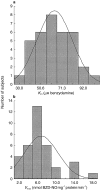
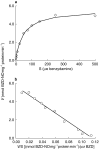
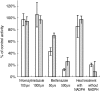
Results: BZD N-oxide formation rates in HLM followed Michaelis-Menten kinetics (mean Km = 64.0 microM, mean Vmax = 6.9 nmol mg-1 protein min-1; n = 35). N-benzylimidazole, a nonspecific CYP inhibitor, and various CYP isoform selective inhibitors did not affect BZD N-oxidation. In contrast, formation of BZD N-oxide was almost abolished by heat treatment of microsomes in the absence of NADPH and strongly inhibited by methimazole, a competitive FMO inhibitor. Recombinant FMO3 and FMO1 (which is not expressed in human liver), but not FMO5, showed BZD N-oxidase activity. Respective Km values for FMO3 and FMO1 were 40.4 microM and 23.6 microM, and respective Vmax values for FMO3 and FMO1 were 29.1 and 40.8 nmol mg-1 protein min-1. Human liver samples (n = 35) were analysed for six known FMO3 polymorphisms. The variants I66M, P135L and E305X were not detected. Samples homozygous for the K158 variant showed significantly reduced Vmax values (median 2.7 nmol mg-1 protein min-1) compared to the carriers of at least one wild type allele (median 6.2 nmol mg-1 protein min-1) (P < 0.05, Mann-Whitney-U-test). The V257M and E308G substitutions had no effect on enzyme activity.
Conclusions: BZD N-oxidation in human liver is mainly catalysed by FMO3 and enzyme activity is affected by FMO3 genotype. BZD may be used as a model substrate for human liver FMO3 activity in vitro and may be further developed as an in vivo probe reflecting FMO3 activity.
Figures

References
-
- Poulsen LL, Ziegler DM. Multisubstrate flavin-containing monooxygenases: applications of mechanism to specificity. Chem Biol Interact. 1995;96:57–73. - PubMed
-
- Ziegler DM. Recent studies on the structure and function of multisubstrate flavin- containing monooxygenases. Annu Rev Pharmacol Toxicol. 1993;33:179–199. - PubMed
-
- Ziegler DM. Flavin-containing monooxygenases: catalytic mechanism and substrate specificities. Drug Metab Rev. 1988;19:1–32. - PubMed
-
- Cashman JR, Park SB, Berkman CE, Cashman LE. Role of hepatic flavin-containing monooxygenase 3 in drug and chemical metabolism in adult humans. Chem Biol Interact. 1995;96:33–46. - PubMed
-
- Lawton MP, Cashman JR, Cresteil T, et al. A nomenclature for the mammalian flavin-containing monooxygenase gene family based on amino acid sequence identities. Arch Biochem Biophys. 1994;308:254–257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

