Effect of rifampicin on the pharmacokinetics and pharmacodynamics of glimepiride
- PMID: 11136298
- PMCID: PMC2015006
- DOI: 10.1046/j.1365-2125.2000.00295.x
Effect of rifampicin on the pharmacokinetics and pharmacodynamics of glimepiride
Abstract
Aims: To study the effects of rifampicin on the pharmacokinetics and pharmaco-dynamics of glimepiride, a new sulphonylurea antidiabetic drug.
Methods: In this randomised, two-phase cross-over study, 10 healthy volunteers were treated for 5 days with 600 mg rifampicin or placebo once daily. On day 6, a single oral dose of 1 mg glimepiride was administered. Plasma glimepiride and blood glucose concentrations were measured up to 12 h.
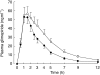
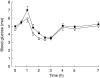
Results: Rifampicin decreased the mean area under the plasma concentration-time curve of glimepiride by 34% (P < 0.001) and the mean elimination half-life by 25% (P < 0.05). No significant differences in the blood glucose response to glimepiride were observed between the placebo and rifampicin phases. However, symptomatic hypoglycaemia occurred only during the placebo phase.
Conclusions: The effects of rifampicin on the pharmacokinetics of glimepiride suggest that rifampicin induced the CYP2C9-mediated metabolism of glimepiride and thereby slightly increased its systemic clearance. Because the interaction was modest and did not significantly alter the glucose-lowering effect of glimepiride in healthy volunteers, it is probably of limited clinical significance. However, in some patients the hypoglycaemic effect of glimepiride may be reduced during concomitant treatment with rifampicin.
Figures

Similar articles
-
Effects of fluconazole and fluvoxamine on the pharmacokinetics and pharmacodynamics of glimepiride.Clin Pharmacol Ther. 2001 Apr;69(4):194-200. doi: 10.1067/mcp.2001.114229. Clin Pharmacol Ther. 2001. PMID: 11309547 Clinical Trial.
-
Effect of rifampin on the pharmacokinetics and pharmacodynamics of gliclazide.Clin Pharmacol Ther. 2003 Oct;74(4):334-40. doi: 10.1016/S0009-9236(03)00221-2. Clin Pharmacol Ther. 2003. PMID: 14534520 Clinical Trial.
-
Effects of rifampin on the pharmacokinetics and pharmacodynamics of glyburide and glipizide.Clin Pharmacol Ther. 2001 Jun;69(6):400-6. doi: 10.1067/mcp.2001.115822. Clin Pharmacol Ther. 2001. PMID: 11406737 Clinical Trial.
-
Glimepiride. A review of its use in the management of type 2 diabetes mellitus.Drugs. 1998 Apr;55(4):563-84. doi: 10.2165/00003495-199855040-00007. Drugs. 1998. PMID: 9561345 Review.
-
Pharmacokinetic basis for the safety of glimepiride in risk groups of NIDDM patients.Horm Metab Res. 1996 Sep;28(9):434-9. doi: 10.1055/s-2007-979833. Horm Metab Res. 1996. PMID: 8911979 Review.
Cited by
-
A Review of CYP-Mediated Drug Interactions: Mechanisms and In Vitro Drug-Drug Interaction Assessment.Biomolecules. 2024 Jan 12;14(1):99. doi: 10.3390/biom14010099. Biomolecules. 2024. PMID: 38254699 Free PMC article. Review.
-
Nephroprotective effect of pioglitazone in a Wistar rat model of adenine‑induced chronic kidney disease.Exp Ther Med. 2024 Aug 7;28(4):392. doi: 10.3892/etm.2024.12681. eCollection 2024 Oct. Exp Ther Med. 2024. PMID: 39161617 Free PMC article.
-
Changes in Host Response to Mycobacterium tuberculosis Infection Associated With Type 2 Diabetes: Beyond Hyperglycemia.Front Cell Infect Microbiol. 2019 Oct 4;9:342. doi: 10.3389/fcimb.2019.00342. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31637222 Free PMC article. Review.
-
Effect of rifampicin on the pharmacokinetics and pharmacodynamics of nateglinide in healthy subjects.Br J Clin Pharmacol. 2003 Oct;56(4):427-32. doi: 10.1046/j.1365-2125.2003.01884.x. Br J Clin Pharmacol. 2003. PMID: 12968988 Free PMC article. Clinical Trial.
-
CYP induction-mediated drug interactions: in vitro assessment and clinical implications.Pharm Res. 2006 Jun;23(6):1089-116. doi: 10.1007/s11095-006-0277-7. Epub 2006 May 26. Pharm Res. 2006. PMID: 16718615 Review.
References
-
- Langtry HD, Balfour JA. Glimepiride. A review of its use in the management of type 2 diabetes mellitus. Drugs. 1998;55:563–584. - PubMed
-
- Venkatesan K. Pharmacokinetic drug interactions with rifampicin. Clin Pharmacokin. 1992;22:47–65. - PubMed
-
- Backman JT, Olkkola KT, Neuvonen PJ. Rifampin drastically reduces plasma concentrations and effects of oral midazolam. Clin Pharmacol Ther. 1996;59:7–13. - PubMed
-
- Strayhorn VA, Baciewicz AM, Self TH. Update on rifampin drug interactions III. Arch Intern Med. 1997;157:2453–2458. - PubMed
-
- Villikka K, Kivistö KT, Backman JT, Olkkola KT, Neuvonen PJ. Triazolam is ineffective in patients taking rifampin. Clin Pharmacol Ther. 1997;61:8–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

