Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines
- PMID: 11136768
- PMCID: PMC87699
- DOI: 10.1128/JCM.39.1.183-190.2001
Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines
Abstract
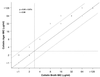
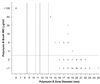
The emergence of infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter spp. has necessitated the search for alternative parenteral agents such as the polymyxins. The National Committee for Clinical Laboratory Standards (NCCLS) documents do not currently provide interpretative criteria for the testing of the polymyxins, colistin and polymyxin B. Therefore, an evaluation of the antimicrobial activity of colistin and polymyxin B was initiated using 200 bloodstream infection pathogens collected through the SENTRY Antimicrobial Surveillance Program. All susceptibility tests were performed according to the NCCLS recommendations. Polymyxin B and colistin displayed a nearly identical spectrum of activity, exhibiting excellent potency against P. aeruginosa (MIC(90), 2 microg/ml) and Acinetobacter sp. (MIC(90), 2 microg/ml). In contrast, they showed limited activity against some other nonfermentative bacilli such as Burkholderia cepacia (MIC(90), >/=128 microg/ml). Excellent correlation was achieved between broth microdilution and agar dilution tests (r = 0.96 to 0.98); 94.3% of the results were +/-1 log(2) dilution between the methods used for both compounds. At a resistance breakpoint of >/=4 microg/ml for both agents, unacceptable false-susceptible or very major errors were noted for colistin (5%) and polymyxin B (6%). Modified zone criteria for colistin (</=11 and >/=14 mm) and polymyxin B (</=10 and >/=14 mm) were suggested, but some degree of error persisted (>/=3.5%). It is recommended that all susceptible disk diffusion results be confirmed by MIC tests using the preferred reference NCCLS method. The quality control (QC) ranges listed in the product package insert require an adjusted range by approximately 3 mm for both NCCLS gram-negative quality control strains. This evaluation of in vitro susceptibility test methods for the polymyxin class drugs confirmed continued serious testing error with the disk diffusion method, the possible need for breakpoint adjustments, and the recalculation of disk diffusion QC ranges. Clinical laboratories should exclusively use MIC methods to assist the therapeutic application of colistin or polymyxin B until disk diffusion test modifications are sanctioned and published by the NCCLS.
Figures

Similar articles
-
Comparison of disc diffusion, Etest and broth microdilution for testing susceptibility of carbapenem-resistant P. aeruginosa to polymyxins.Ann Clin Microbiol Antimicrob. 2007 Aug 15;6:8. doi: 10.1186/1476-0711-6-8. Ann Clin Microbiol Antimicrob. 2007. PMID: 17697363 Free PMC article.
-
Differences in potency and categorical agreement between colistin and polymyxin B when testing 15,377 clinical strains collected worldwide.Diagn Microbiol Infect Dis. 2015 Dec;83(4):379-81. doi: 10.1016/j.diagmicrobio.2015.08.013. Epub 2015 Aug 28. Diagn Microbiol Infect Dis. 2015. PMID: 26415906
-
Evaluation of susceptibility testing methods for polymyxin.Int J Infect Dis. 2010 Jul;14(7):e596-601. doi: 10.1016/j.ijid.2009.09.001. Epub 2009 Dec 31. Int J Infect Dis. 2010. PMID: 20045367
-
Antimicrobial Susceptibility Testing for Polymyxins: Challenges, Issues, and Recommendations.J Clin Microbiol. 2019 Mar 28;57(4):e01390-18. doi: 10.1128/JCM.01390-18. Print 2019 Apr. J Clin Microbiol. 2019. PMID: 30541939 Free PMC article. Review.
-
Susceptibility testing of the polymyxins: where are we now?Pharmacotherapy. 2015 Jan;35(1):22-7. doi: 10.1002/phar.1505. Epub 2014 Oct 20. Pharmacotherapy. 2015. PMID: 25329490 Review.
Cited by
-
Antibiotic susceptibility pattern and identification of extended spectrum β-lactamases (ESBLs) in clinical isolates of Klebsiella pneumoniae from Shiraz, Iran.Iran J Microbiol. 2016 Feb;8(1):55-61. Iran J Microbiol. 2016. PMID: 27092225 Free PMC article.
-
Refined medium for direct isolation of Vibrio vulnificus from oyster tissue and seawater.Appl Environ Microbiol. 2007 May;73(9):3098-100. doi: 10.1128/AEM.02245-06. Epub 2007 Mar 2. Appl Environ Microbiol. 2007. PMID: 17337558 Free PMC article.
-
Polymyxin B, in combination with fluconazole, exerts a potent fungicidal effect.J Antimicrob Chemother. 2010 May;65(5):931-8. doi: 10.1093/jac/dkq046. Epub 2010 Feb 18. J Antimicrob Chemother. 2010. PMID: 20167587 Free PMC article.
-
Antimicrobial resistance, class 1 integrons, and genomic island 1 in Salmonella isolates from Vietnam.PLoS One. 2010 Feb 26;5(2):e9440. doi: 10.1371/journal.pone.0009440. PLoS One. 2010. PMID: 20195474 Free PMC article.
-
Multicity outbreak of carbapenem-resistant Acinetobacter baumannii isolates producing the carbapenemase OXA-40.Antimicrob Agents Chemother. 2006 Sep;50(9):2941-5. doi: 10.1128/AAC.00116-06. Antimicrob Agents Chemother. 2006. PMID: 16940085 Free PMC article.
References
-
- Appleman M D, Belzberg H, Citron D M, Heseltine P N, Yellin A E, Murray J, Berne T V. In vitro activities of nontraditional antimicrobials against multiresistant Acinetobacter baumannii strains isolated in an intensive care unit outbreak. Antimicrob Agents Chemother. 2000;44:1035–1040. - PMC - PubMed
-
- Barry A L. The antimicrobic susceptibility test: principle and practices. New York, N.Y: Lea & Febiger; 1976. Establishment of zone-size interpretative criteria; p. 196.
-
- Catchpole C R, Andrews J M, Brenwald N, Wise R. A reassessment of the in vitro activity of colistin sulphomethate. J Antimicrob Chemother. 1997;39:255–260. - PubMed
-
- Evans M E, Feola D J, Rapp R P. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother. 1999;33:960–967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

