A regulatory role for Src homology 2 domain-containing inositol 5'-phosphatase (SHIP) in phagocytosis mediated by Fc gamma receptors and complement receptor 3 (alpha(M)beta(2); CD11b/CD18)
- PMID: 11136821
- PMCID: PMC2195884
- DOI: 10.1084/jem.193.1.61
A regulatory role for Src homology 2 domain-containing inositol 5'-phosphatase (SHIP) in phagocytosis mediated by Fc gamma receptors and complement receptor 3 (alpha(M)beta(2); CD11b/CD18)
Abstract
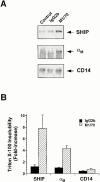
The Src homology 2 domain-containing inositol 5'-phosphatase (SHIP) is recruited to immunoreceptor tyrosine-based inhibition motif (ITIM)-containing proteins, thereby suppressing phosphatidylinositol 3-kinase (PI 3-kinase)-dependent pathways. The role of SHIP in phagocytosis, a PI 3-kinase-dependent pathway, is unknown. Overexpression of SHIP in macrophages led to an inhibition of phagocytosis mediated by receptors for the Fc portion of IgG (Fc gamma Rs). In contrast, macrophages expressing catalytically inactive SHIP or lacking SHIP expression demonstrated enhanced phagocytosis. To determine whether SHIP regulates phagocytosis mediated by receptors that are not known to recruit ITIMs, we determined the effect of SHIP expression on complement receptor 3 (CR3; CD11b/CD18; alpha(M)beta(2))-dependent phagocytosis. Macrophages overexpressing SHIP demonstrated impaired CR3-mediated phagocytosis, whereas macrophages expressing catalytically inactive SHIP demonstrated enhanced phagocytosis. CR3-mediated phagocytosis in macrophages derived from SHIP(-/-) mice was up to 2.5 times as efficient as that observed in macrophages derived from littermate controls. SHIP was localized to Fc gamma R- and CR3-containing phagocytic cups and was recruited to the cytoskeleton upon clustering of CR3. In a transfected COS cell model of activation-independent CR3-mediated phagocytosis, catalytically active but not inactive SHIP also inhibited phagocytosis. We conclude that PI 3-kinase(s) and SHIP regulate multiple forms of phagocytosis and that endogenous SHIP plays a role in modulating beta(2) integrin outside-in signaling.
Figures







References
-
- Cox D., Tseng C.-C., Bjekic G., Greenberg S. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 1999;274:1240–1247. - PubMed
-
- Huber M., Helgason C.D., Damen J.E., Scheid M., Duronio V., Liu L., Ware M.D., Humphries R.K., Krystal G. The role of SHIP in growth factor induced signalling. Prog. Biophys. Mol. Biol. 1999;71:423–434. - PubMed
-
- Rohrschneider L.R., Fuller J.F., Wolf I., Liu Y., Lucas D.M. Structure, function, and biology of SHIP proteins. Genes Dev. 2000;14:505–520. - PubMed
-
- Ono M., Okada H., Bolland S., Yanagi S., Kurosaki T., Ravetch J.V. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

