Mucosal immunity against parasitic gastrointestinal nematodes
- PMID: 11138315
- PMCID: PMC2721204
- DOI: 10.3347/kjp.2000.38.4.209
Mucosal immunity against parasitic gastrointestinal nematodes
Abstract
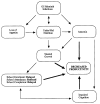
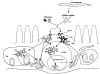
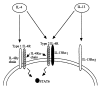
The last two decades witnessed significant advances in the efforts of immunoparasitologists to elucidate the nature and role of the host mucosal defence mechanisms against intestinal nematode parasites. Aided by recent advances in basic immunology and biotechnology with the concomitant development of well defined laboratory models of infection, immunoparasitologists have more precisely analyzed and defined the different immune effector mechanisms during the infection; resulting in great improvement in our current knowledge and understanding of protective immunity against gastrointestinal (GI) nematode parasites. Much of this current understanding comes from experimental studies in laboratory rodents, which have been used as models of livestock and human GI nematode infections. These rodent studies, which have concentrated on Heligmosomoides polygyrus, Nippostrongylus brasiliensis, Strongyloides ratti/S. venezuelensis, Trichinella spiralis and Trichuris muris infections in mice and rats, have helped in defining the types of T cell responses that regulate effector mechanisms and the effector mechanisms responsible for worm expulsion. In addition, these studies bear indications that traditionally accepted mechanisms of resistance such as eosinophilia and IgE responses may not play as important roles in protection as were previously conceived. In this review, we shall, from these rodent studies, attempt an overview of the mucosal and other effector responses against intestinal nematode parasites beginning with the indices of immune protection as a model of the protective immune responses that may occur in animals and man.
Figures

Similar articles
-
Intestinal nematode parasites, cytokines and effector mechanisms.Int J Parasitol. 1998 Aug;28(8):1145-58. doi: 10.1016/s0020-7519(98)00087-3. Int J Parasitol. 1998. PMID: 9762559 Review.
-
Prospects for the immunological control of ruminant gastrointestinal nematodes: natural immunity, can it be harnessed?Int J Parasitol. 1996 Aug-Sep;26(8-9):801-11. doi: 10.1016/s0020-7519(96)80044-0. Int J Parasitol. 1996. PMID: 8923129 Review.
-
Responses of inbred mouse strains to infection with intestinal nematodes.J Helminthol. 2003 Jun;77(2):119-24. doi: 10.1079/JOH2003175. J Helminthol. 2003. PMID: 12756065
-
Importance of immunoglobulin E (IgE) in the protective mechanism against gastrointestinal nematode infection: looking at the intestinal mucosae.Rev Inst Med Trop Sao Paulo. 2001 Sep-Oct;43(5):291-9. doi: 10.1590/s0036-46652001000500011. Rev Inst Med Trop Sao Paulo. 2001. PMID: 11696854 Review.
-
Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models.Annu Rev Immunol. 1997;15:505-33. doi: 10.1146/annurev.immunol.15.1.505. Annu Rev Immunol. 1997. PMID: 9143698 Review.
Cited by
-
Intestinal strongyloidiasis and hyperinfection syndrome.Clin Mol Allergy. 2006 May 30;4:8. doi: 10.1186/1476-7961-4-8. Clin Mol Allergy. 2006. PMID: 16734908 Free PMC article.
-
Effects of anti-allergic drugs on intestinal mastocytosis and worm expulsion of rats infected with Neodiplostomum seoulense.Korean J Parasitol. 2003 Jun;41(2):81-7. doi: 10.3347/kjp.2003.41.2.81. Korean J Parasitol. 2003. PMID: 12815318 Free PMC article.
-
Goats are more susceptible to Haemonchus contortus infection than sheep under similar experimental settings.Sci Rep. 2024 Oct 25;14(1):25379. doi: 10.1038/s41598-024-74112-1. Sci Rep. 2024. PMID: 39455578 Free PMC article.
-
Strongyloidiasis in Assam, India: A community-based study.Trop Parasitol. 2011 Jan;1(1):30-2. doi: 10.4103/2229-5070.72110. Trop Parasitol. 2011. PMID: 23508997 Free PMC article.
-
Intestinal histopathology and in situ postures of Gymnophalloides seoi in experimentally infected mice.Korean J Parasitol. 2001 Mar;39(1):31-41. doi: 10.3347/kjp.2001.39.1.31. Korean J Parasitol. 2001. PMID: 11301588 Free PMC article.
References
-
- Abe T, Khan WI, Sugaya H, Ishida K, Yoshimura K. Prolongation of infection time and failure of restoring fecundity of mouse-nonadaptive Nippostrongylus brasiliensis by administration of cyclophosphamide or anti-CD4 antibody in mice. Jpn J Parasitol. 1994;43:288–293.
-
- Abe T, Nawa Y. Reconstitution of mucosal mast cells in W/Wv mice by adoptive transfer of bone marrow-derived cultured mast cells and its effects on the protective capacity to Strongyloides ratti infection. Parasite Immunol. 1987a;9:31–38. - PubMed
-
- Abe T, Nawa Y. Localization of mucosal mast cells in W/Wv mice after reconstitution with bone marrow cells or cultured mast cells, and its relation to the protective capacity to Strongyloides infection. Parasite Immunol. 1987b;9:477–485. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

