Angiotensin-converting enzyme (ACE) inhibition attenuates insulin-like growth factor-I (IGF-I) induced cardiac fibroblast proliferation
- PMID: 11139436
- PMCID: PMC1572499
- DOI: 10.1038/sj.bjp.0703740
Angiotensin-converting enzyme (ACE) inhibition attenuates insulin-like growth factor-I (IGF-I) induced cardiac fibroblast proliferation
Abstract
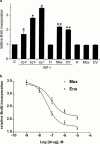
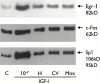
The effects of angiotensin-converting enzyme (ACE) inhibition and angiotensin type 1 (AT(1)) receptor blockade on insulin-like growth factor-I (IGF-I) induced proliferation and immediate-early-gene expression of neonatal rat cardiac fibroblasts were investigated. Moreover the role of the IGF-I receptor (IGF-IR) in this process was evaluated. IGF-I (10(-9) - 10(-7) M) stimulated neonatal rat cardiac fibroblast growth in a dose-dependent fashion (maximum: 3.5+/-0.1 fold, 10(-7) M), as determined by 5-bromo-2'-deoxyuridine (BrdU) incorporation. ACE inhibition or AT(1) receptor blockade attenuated the IGF-I (10(-7) M) induced neonatal rat cardiac fibroblast growth in a concentration-dependent fashion (moexiprilat: 50+/-2%, enalaprilat: 31+/-2%, CV11974; 58+/-1%, all: 10(-7) M). IGF-I stimulated cellular growth was accompanied by an upregulation of the immediate early genes c-Fos (2.4+/-0.3 fold), Egr-1 (4.7+/-1.1 fold) and Sp1 (6.2+/-0.7 fold). IGF-I induced expression was completely inhibited by ACE inhibition or AT(1) receptor blockade. Stimulation with IGF-I or Ang II (10(-7) M) increased IGF-IR expression 5.7+/-0. 5 fold and 3.6+/-0.5 fold respectively. The IGF-I induced overexpression of the IGF-IR was reduced by ACE inhibition with moexiprilat (10(-7) M) by 79+/-7% and by AT(1) receptor blockade with CV11974 (10(-7) M) by 79+/-5%. These data demonstrate that the mitogenic action of IGF-I in neonatal rat cardiac fibroblasts is in part mediated by activation of the renin-angiotensin system (RAS) with subsequent upregulation of IGF-IR expression. This observation has important implications for the treatment of cardiac diseases with ACE inhibitors alone and their combination with IGF-I or growth hormone.
Figures
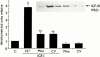

Similar articles
-
Angiotensin converting enzyme inhibitors block mitogenic signalling pathways in rat cardiac fibroblasts.Naunyn Schmiedebergs Arch Pharmacol. 1999 May;359(5):394-9. doi: 10.1007/pl00005366. Naunyn Schmiedebergs Arch Pharmacol. 1999. PMID: 10498289
-
Effects of moexiprilat on oestrogen-stimulated cardiac fibroblast growth.Br J Pharmacol. 1997 Aug;121(7):1350-4. doi: 10.1038/sj.bjp.0701263. Br J Pharmacol. 1997. PMID: 9257913 Free PMC article.
-
Angiotensin converting enzyme inhibition modulates cardiac fibroblast growth.J Hypertens. 1998 Mar;16(3):377-84. doi: 10.1097/00004872-199816030-00015. J Hypertens. 1998. PMID: 9557931
-
Modulation of the expression of early genes by polyunsaturated fatty acids.Prostaglandins Leukot Essent Fatty Acids. 1997 Oct;57(4-5):353-7. doi: 10.1016/s0952-3278(97)90410-5. Prostaglandins Leukot Essent Fatty Acids. 1997. PMID: 9430378 Review.
-
The biological role of truncated insulin-like growth factor-1 and the tripeptide GPE in the central nervous system.Ann N Y Acad Sci. 1993 Aug 27;692:183-91. doi: 10.1111/j.1749-6632.1993.tb26216.x. Ann N Y Acad Sci. 1993. PMID: 8215022 Review. No abstract available.
Cited by
-
Expression, regulation and function of Egr1 during implantation and decidualization in mice.Cell Cycle. 2014;13(16):2626-40. doi: 10.4161/15384101.2014.943581. Cell Cycle. 2014. PMID: 25486203 Free PMC article.
-
Cardiac-specific IGF-1 receptor transgenic expression protects against cardiac fibrosis and diastolic dysfunction in a mouse model of diabetic cardiomyopathy.Diabetes. 2010 Jun;59(6):1512-20. doi: 10.2337/db09-1456. Epub 2010 Mar 9. Diabetes. 2010. PMID: 20215428 Free PMC article.
-
Renin-Angiotensin System Blockade Improves Cardiac Indices in Acromegaly Patients.Exp Clin Endocrinol Diabetes. 2017 Jun;125(6):365-367. doi: 10.1055/s-0042-123710. Epub 2017 Feb 6. Exp Clin Endocrinol Diabetes. 2017. PMID: 28166592 Free PMC article. Clinical Trial.
-
Additive interaction between the renin-angiotensin system and lipid metabolism for cancer in type 2 diabetes.Diabetes. 2009 Jul;58(7):1518-25. doi: 10.2337/db09-0105. Epub 2009 Apr 28. Diabetes. 2009. PMID: 19401427 Free PMC article.
-
Use of ACE inhibitors is associated with elevated levels of IGFBP-3 among hypertensive older adults: results from the IlSIRENTE study.Eur J Clin Pharmacol. 2007 Apr;63(4):389-95. doi: 10.1007/s00228-007-0262-z. Epub 2007 Feb 2. Eur J Clin Pharmacol. 2007. PMID: 17273834
References
-
- ANWAR A., ZAHID A.A., PHILLIPS L., DELAFONTAINE P. Insulin-like growth factor binding protein-4 expression is decreased by angiotensin II and thrombin in rat aortic vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2000;20:370–376. - PubMed
-
- BROWN N.J., VAUGHAN D.E. Angiotensin-converting enzyme inhibitors. Circulation. 1990;97:1411–1420. - PubMed
-
- BRINK M., CHRAST J., PRICE S.R., MTICH W.E., DELAFONTAINE P. Angiotensin II stimulates gene expression of cardiac insulin-like growth factor I and its receptor through effects on blood pressure and food intake. Hypertension. 1999;34:1053–1059. - PubMed
-
- CLARK R. Growth hormone and Insulin like growth factor 1: New endocrine therapies in cardiology. Trend. Cardiovasc. Med. 1997;7:264–268. - PubMed
-
- DELAFONTAINE P., LOU H. Angiotensin II regulates insulin-like growth factor I gene expression in vascular smooth muscle cells. J. Biol. Chem. 1993;268:16866–16870. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

