Selective phenylalkylamine block of I(Kr) over other K(+) currents in guinea-pig ventricular myocytes
- PMID: 11139462
- PMCID: PMC1572516
- DOI: 10.1038/sj.bjp.0703758
Selective phenylalkylamine block of I(Kr) over other K(+) currents in guinea-pig ventricular myocytes
Abstract
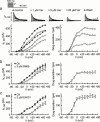
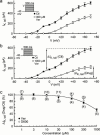
Previous studies on verapamil and D600 have established that the Ca(2+)-channel blockers also inhibit delayed-rectifier K(+) currents in cardiac tissues and myocytes. However, estimated IC(50) values range over two to three orders of concentration, and it is unclear whether this reflects a high selectivity by one or both of the phenylalkylamines for particular K(+) channels. The purpose of the present study was to determine the concentration-dependent actions of verapamil and D600 on three defined cardiac K(+) currents. Guinea-pig ventricular myocytes in the conventional whole-cell configuration were bathed with normal Tyrode's or K(+)-free solution, and pulsed from -80 mV for measurement of the effects of 0.01 microM to 3 mM verapamil and D600 on the inwardly-rectifying K(+) current (I:(Kl)) and the two delayed-rectifier K(+) currents, rapidly-activating I:(Kr) and slowly-activating I:(Ks). The phenylalkylamines inhibited both inward- and outward-directed I:(Kl). The IC(50) values for outward I:(Kl) were approximately 220 microM. Verapamil and D600 were approximately equipotent inhibitors of the delayed-rectifier K(+) currents. They inhibited I:(Kr) with IC(50) near 3 microM, and I:(Ks) with IC(50) > or =280 microM. These results are discussed in relation to previous findings on K(+) currents and to the clinical actions of the drugs.
Figures





Similar articles
-
Block of cardiac delayed-rectifier and inward-rectifier K+ currents by nisoldipine.Br J Pharmacol. 2003 Nov;140(5):863-70. doi: 10.1038/sj.bjp.0705518. Epub 2003 Oct 6. Br J Pharmacol. 2003. PMID: 14530219 Free PMC article.
-
Low-affinity block of cardiac K(+) currents by nifedipine.Eur J Pharmacol. 2000 Aug 4;401(2):137-43. doi: 10.1016/s0014-2999(00)00413-1. Eur J Pharmacol. 2000. PMID: 10924918
-
Bepridil differentially inhibits two delayed rectifier K(+) currents, I(Kr) and I(Ks), in guinea-pig ventricular myocytes.Br J Pharmacol. 1999 Dec;128(8):1733-8. doi: 10.1038/sj.bjp.0702959. Br J Pharmacol. 1999. PMID: 10588929 Free PMC article.
-
Differences in the effects of urinary incontinence agents S-oxybutynin and terodiline on cardiac K(+) currents and action potentials.Br J Pharmacol. 2000 Sep;131(2):245-54. doi: 10.1038/sj.bjp.0703595. Br J Pharmacol. 2000. PMID: 10991917 Free PMC article.
-
Pharmacology of cardiac potassium channels.Adv Pharmacol. 2010;59:93-134. doi: 10.1016/S1054-3589(10)59004-5. Adv Pharmacol. 2010. PMID: 20933200 Review.
Cited by
-
Verapamil inhibits Kir2.3 channels by binding to the pore and interfering with PIP2 binding.Naunyn Schmiedebergs Arch Pharmacol. 2023 Apr;396(4):659-667. doi: 10.1007/s00210-022-02342-z. Epub 2022 Nov 29. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 36445385 Free PMC article.
References
-
- BARROS F., DELGADO L.M., DEL CAMINO D., DE LA PENA P. Characteristics and modulation by thyrotropin-releasing hormone of an inwardly rectifying K+ current in patch-perforated GH3 anterior pituitary cells. Pflügers Arch. 1992;422:31–39. - PubMed
-
- BAYER R., KALUSCHE D., KAUFMANN R., MANNHOLD R. Inotropic and electrophysiological actions of verapamil and D 600 in mammalian myocardium. III. Effects of the optical isomers on transmembrane action potentials. Naunyn Schmiedeberg's Arch. Pharmacol. 1975;290:81–97. - PubMed
-
- CHOUABE C., DRICI M.D., ROMEY G., BARHANIN J., LAZDUNSKI M. HERG and KvLQT1/IsK, the cardiac K+ channels involved in long QT syndromes, are targets for calcium channel blockers. Mol. Pharmacol. 1998;54:695–703. - PubMed
-
- CRANEFIELD P.F., ARONSON R.S., WIT A.L. Effect of verapamil on the normal action potential and on a calcium-dependent slow response of canine cardiac Purkinje fibers. Circ. Res. 1974;34:204–213. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

