Muscle cachexia: current concepts of intracellular mechanisms and molecular regulation
- PMID: 11141219
- PMCID: PMC1421177
- DOI: 10.1097/00000658-200101000-00003
Muscle cachexia: current concepts of intracellular mechanisms and molecular regulation
Abstract
Objective: To review present knowledge of intracellular mechanisms and molecular regulation of muscle cachexia.
Summary background data: Muscle cachexia, mainly reflecting degradation of myofibrillar proteins, is an important clinical feature in patients with severe injury, sepsis, and cancer. The catabolic response in skeletal muscle may result in muscle wasting and weakness, delaying or preventing ambulation and rehabilitation in these patients and increasing the risk for pulmonary complications.
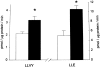
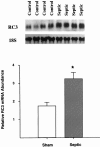
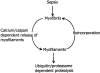
Results: Muscle cachexia, induced by severe injury, sepsis, and cancer, is associated with increased gene expression and activity of the calcium/calpain- and ubiquitin/proteasome-proteolytic pathways. Calcium/calpain-regulated release of myofilaments from the sarcomere is an early, and perhaps rate-limiting, component of the catabolic response in muscle. Released myofilaments are ubiquitinated in the N-end rule pathway, regulated by the ubiquitin-conjugating enzyme E2(14k) and the ubiquitin ligase E3 alpha, and degraded by the 26S proteasome.
Conclusions: An understanding of the mechanisms regulating muscle protein breakdown is important for the development of therapeutic strategies aimed at treating or preventing muscle cachexia in patients with severe injury, sepsis, cancer, and perhaps other catabolic conditions as well.
Figures



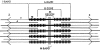


References
-
- Rosenblatt S, Clowes GHA, George BC,et al. Exchange of amino acids by muscle and liver in sepsis. Comparative studies in vivo and in vitro. Arch Surg 1983; 118: 167–175. - PubMed
-
- Windmueller HG, Spaeth AE. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. J Biol Chem 1980; 255: 107–112. - PubMed
-
- Newsholme EA, Parry-Billings M. Properties of glutamine release from muscle and its importance for the immune system. J Parenteral Enteral Nutr 1990; 14: 63S–67S. - PubMed
-
- Reid WD, MacGowan NA. Respiratory muscle injury in animal models and humans. Mol Cell Biochem 1998; 179: 63–80. - PubMed
-
- Andreyev HJN, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 1998; 34: 503–509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

