Three-dimensional imaging of embryonic mouse kidney by two-photon microscopy
- PMID: 11141478
- PMCID: PMC1850252
- DOI: 10.1016/S0002-9440(10)63943-0
Three-dimensional imaging of embryonic mouse kidney by two-photon microscopy
Abstract
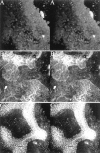
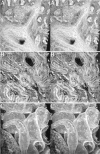
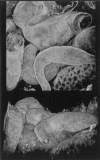
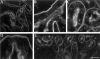
Developing mammalian embryonic kidney becomes progressively more elaborate as the ureteric bud branches into undifferentiated mesenchyme. Morphological perturbations of nephrogenesis, such as those seen in inherited renal diseases or induced in transgenic animals, require careful and often tedious documentation by multiple methodologies. We have applied a relatively quick and simple approach combining two-photon microscopy and advanced three-dimensional (3-D) imaging techniques to visualize and evaluate these complex events. As compared with laser confocal microscopy, two-photon microscopy offers superior optical sectioning deep into biological tissues, permitting analysis of large, heterogeneous, 3-D structures such as developing mouse kidney. Embryonic and newborn mouse kidneys were fluorescently labeled with lectins, phalloidin, or antibody. Three-dimensional image volumes were then collected. The resulting volume data sets were processed using a novel 3-D visualization technique. Reconstructed image volumes demonstrate the dichotomous branching of ureteric bud as it progresses from a simple, symmetrical structure into an elaborate, asymmetrical collecting system of multiple branches. Detailed morphology of in situ cysts was elucidated in a transgene-induced mouse model of polycystic kidney disease. We expect this integration of two-photon microscopy with advanced 3-D image analysis will provide a powerful tool for illuminating a variety of complex developmental processes in multiple dimensions.
Figures

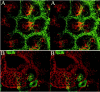
References
-
- Inoue S: Foundations of confocal scanned imaging in light microscopy. Pawley JB eds. Handbook of Biological Microscopy. 1995, :pp 1-17 Plenum Press, New York
-
- Keller HE: Objective lenses for confocal microscopy. Pawley JB eds. Handbook of Biological Microscopy. 1995, :pp 111-126 Plenum Press, New York
-
- Dunn KW, Wang E: Optical aberrations and objective choice in multicolor confocal microscopy. Biotechniques 2000, 28:542-550 - PubMed
-
- Denk W, Piston DW, Webb WW: Two-photon molecular excitation in laser-scanning microscopy. Pawley JB eds. Handbook of Biological Confocal Microscopy. 1995, :pp 445-458 Plenum Press, New York
-
- Davies JA, Bard JBL: Inductive interactions between mesenchyme and the ureteric bud. Exp Nephrol 1996, 4:77-85 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

