Abeta-induced inflammatory processes in microglia cells of APP23 transgenic mice
- PMID: 11141480
- PMCID: PMC1850262
- DOI: 10.1016/s0002-9440(10)63945-4
Abeta-induced inflammatory processes in microglia cells of APP23 transgenic mice
Abstract
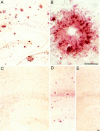
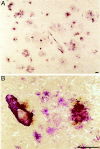
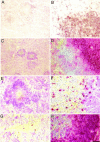
A microglial response is part of the inflammatory processes in Alzheimer's disease (AD). We have used APP23 transgenic mice overexpressing human amyloid precursor protein with the Swedish mutation to characterize this microglia response to amyloid deposits in aged mice. Analyses with MAC-1 and F4/80 antibodies as well as in vivo labeling with bromodeoxyuridine demonstrate that microglia in the plaque vicinity are in an activated state and that proliferation contributes to their accumulation at the plaque periphery. The amyloid-induced microglia activation may be mediated by scavenger receptor A, which is generally elevated, whereas the increased immunostaining of the receptor for advanced glycation end products is more restricted. Although components of the phagocytic machinery such as macrosialin and Fc receptors are increased in activated microglia, efficient clearance of amyloid is missing seemingly because of the lack of amyloid-bound autoantibodies. Similarly, although up-regulation of major histocompatibility complex class II (IA) points toward an intact antigen-presenting function of microglia, lack of T and B lymphocytes does not indicate a cell-mediated immune response in the brains of APP23 mice. The similar characteristics of microglia in the APP23 mice and in AD render the mouse model suitable to study the role of inflammatory processes during AD pathogenesis.
Figures



References
-
- Stewart WF, Kawas C, Corrada M, Meter EJ: Risk of Alzheimer’s disease and duration of NSAID use. Neurology 1997, 48:626-632 - PubMed
-
- McGeer PL, Schulzer M, McGeer EG: Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology 1996, 47:425-432 - PubMed
-
- Mackenzie IR, Hao C, Munoz DG: Role of microglia in senile plaque formation. Neurobiol Aging 1995, 16:797-804 - PubMed
-
- McGeer PL, McGeer EG: The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Rev 1995, 21:195-218 - PubMed
-
- Eikelenboom P, Zhan SS, Van Gool WA, Allsop D: Inflammatory mechanisms in Alzheimer’s disease. Trends Pharmacol Sci 1994, 15:447-450 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

