Leukocyte-mediated endothelial cell injury and death in the diabetic retina
- PMID: 11141487
- PMCID: PMC1850259
- DOI: 10.1016/S0002-9440(10)63952-1
Leukocyte-mediated endothelial cell injury and death in the diabetic retina
Abstract
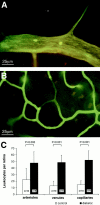
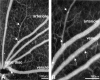
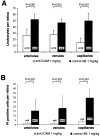
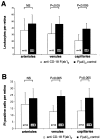
Endothelial cell death is a hallmark of diabetic retinopathy. Its occurrence is required for the formation of acellular (devitalized) capillaries, lesions that produce irreversible retinal ischemia through their inability to support blood flow. The mechanisms underlying diabetic retinal endothelial cell injury and death remain largely unknown. The current study demonstrates that adherent leukocytes are temporally and spatially associated with retinal endothelial cell injury and death within 1 week of streptozotocin-induced experimental diabetes in rats. Moreover, the antibody-based neutralization of intercellular adhesion molecule-1 and CD18 is shown to prevent both leukocyte adhesion and retinal endothelial cell injury and death. These data highlight the central and causal role of adherent leukocytes in the pathogenesis of diabetic retinopathy. They also underscore the potential utility of anti-intercellular adhesion molecule1- and anti-CD18-based therapies in the treatment of diabetic retinopathy, a newly recognized inflammatory disease.
Figures


References
-
- Adamis AP, Shima DT, Tolentino M, Gragoudas ES, Ferrara N, Folkman J, D’Amore PA, Miller JW: Inhibition of VEGF prevents retinal ischemia-associated iris neovascularization in a primate. Arch Ophthalmol 1996, 114:66-71 - PubMed
-
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, Ferrara N, King G, Smith LEH: Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA 1995, 92:10457-10461 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

