Functional characterization of Xenopus small heat shock protein, Hsp30C: the carboxyl end is required for stability and chaperone activity
- PMID: 11147966
- PMCID: PMC312903
- DOI: 10.1379/1466-1268(2000)005<0148:fcoxsh>2.0.co;2
Functional characterization of Xenopus small heat shock protein, Hsp30C: the carboxyl end is required for stability and chaperone activity
Abstract
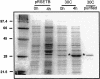
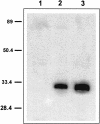
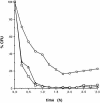
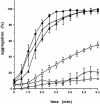
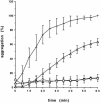
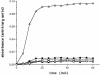
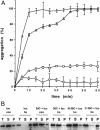
Small heat shock proteins protect cells from stress presumably by acting as molecular chaperones. Here we report on the functional characterization of a developmentally regulated, heat-inducible member of the Xenopus small heat shock protein family, Hsp30C. An expression vector containing the open reading frame of the Hsp30C gene was expressed in Escherichia coli. These bacterial cells displayed greater thermoresistance than wild type or plasmid-containing cells. Purified recombinant protein, 30C, was recovered as multimeric complexes which inhibited heat-induced aggregation of either citrate synthase or luciferase as determined by light scattering assays. Additionally, 30C attenuated but did not reverse heat-induced inactivation of enzyme activity. In contrast to an N-terminal deletion mutant, removal of the last 25 amino acids from the C-terminal end of 30C severely impaired its chaperone activity. Furthermore, heat-treated concentrated solutions of the C-terminal mutant formed nonfunctional complexes and precipitated from solution. Immunoblot and gel filtration analysis indicated that 30C binds with and maintains the solubility of luciferase preventing it from forming heat-induced aggregates. Coimmunoprecipitation experiments suggested that the carboxyl region is necessary for 30C to interact with target proteins. These results clearly indicate a molecular chaperone role for Xenopus Hsp30C and provide evidence that its activity requires the carboxyl terminal region.
Figures





Similar articles
-
Xenopus small heat shock proteins, Hsp30C and Hsp30D, maintain heat- and chemically denatured luciferase in a folding-competent state.Cell Stress Chaperones. 2002 Jan;7(1):6-16. doi: 10.1379/1466-1268(2002)007<0006:xshsph>2.0.co;2. Cell Stress Chaperones. 2002. PMID: 11892988 Free PMC article.
-
Molecular chaperone function of the Rana catesbeiana small heat shock protein, hsp30.Comp Biochem Physiol A Mol Integr Physiol. 2004 Oct;139(2):175-82. doi: 10.1016/j.cbpb.2004.08.006. Comp Biochem Physiol A Mol Integr Physiol. 2004. PMID: 15528166
-
Mutation or deletion of the C-terminal tail affects the function and structure of Xenopus laevis small heat shock protein, hsp30.Comp Biochem Physiol B Biochem Mol Biol. 2002 Sep;133(1):95-103. doi: 10.1016/s1096-4959(02)00110-0. Comp Biochem Physiol B Biochem Mol Biol. 2002. PMID: 12223216
-
Expression and function of small heat shock protein genes during Xenopus development.Semin Cell Dev Biol. 2003 Oct;14(5):259-66. doi: 10.1016/j.semcdb.2003.09.022. Semin Cell Dev Biol. 2003. PMID: 14986855 Review.
-
Analysis of molecular chaperones using a Xenopus oocyte protein refolding assay.Methods Mol Biol. 2006;322:213-22. doi: 10.1007/978-1-59745-000-3_15. Methods Mol Biol. 2006. PMID: 16739726 Review.
Cited by
-
Withaferin A induces proteasome inhibition, endoplasmic reticulum stress, the heat shock response and acquisition of thermotolerance.PLoS One. 2012;7(11):e50547. doi: 10.1371/journal.pone.0050547. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226310 Free PMC article.
-
Effect of manganese on heat stress protein synthesis of new-born rats.World J Gastroenterol. 2002 Feb;8(1):114-8. doi: 10.3748/wjg.v8.i1.114. World J Gastroenterol. 2002. PMID: 11833084 Free PMC article.
-
The microtubule-associated protein, NUD-1, exhibits chaperone activity in vitro.Cell Stress Chaperones. 2009 Jan;14(1):95-103. doi: 10.1007/s12192-008-0061-1. Epub 2008 Jul 15. Cell Stress Chaperones. 2009. PMID: 18626791 Free PMC article.
-
Oligomeric structure and chaperone-like activity of Drosophila melanogaster mitochondrial small heat shock protein Hsp22 and arginine mutants in the alpha-crystallin domain.Cell Stress Chaperones. 2017 Jul;22(4):577-588. doi: 10.1007/s12192-017-0784-y. Epub 2017 Apr 7. Cell Stress Chaperones. 2017. PMID: 28389817 Free PMC article.
-
Xenopus small heat shock proteins, Hsp30C and Hsp30D, maintain heat- and chemically denatured luciferase in a folding-competent state.Cell Stress Chaperones. 2002 Jan;7(1):6-16. doi: 10.1379/1466-1268(2002)007<0006:xshsph>2.0.co;2. Cell Stress Chaperones. 2002. PMID: 11892988 Free PMC article.
References
-
- Andley UP, Mathur S, Griest TA, Petrash JM. Cloning, expression, and chaperone-like activity of human αA-crystallin. J Biol Chem. 1996;271:31973–31980. - PubMed
-
- Arrigo A-P. Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. J Biol Chem. 1998;379:19–26. - PubMed
-
- Arrigo AP, Landry J 1994 Expression and function of the low-molecular-weight heat shock proteins. In: The biology of heat shock proteins and molecular chaperones, edited by Morimoto RI, Tissieres A, Georgopoulos C. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, p 335–373.
-
- Behlke J, Lutsch G, Gaestel M, Bielka H. Supramolecular structure of the recombinant murine small heat shock protein hsp25. FEBS Lett. 1991;288:119–122. - PubMed
-
- Beissinger M, Buchner J. How chaperones fold proteins. Biol Chem. 1998;379:245–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
