Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses
- PMID: 11148224
- PMCID: PMC2193340
- DOI: 10.1084/jem.193.2.207
Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses
Abstract
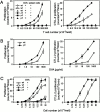
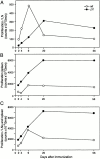
The paucity of lymph node T cells (plt) mutation leads to a loss of CCL21 and CCL19 expression in secondary lymphoid organs. plt mice have defects in the migration of naive T cells and activated dendritic cells into the T cell zones of lymphoid organs, suggesting that they would have defects in T cell immune responses. We now demonstrate T cell responses in plt mice are delayed but ultimately enhanced. Responses to contact sensitization are decreased at day 2 after priming but increased at day 6. After subcutaneous immunization, antigen-specific T cell proliferation and cytokine production in plt mice are increased and remain markedly elevated for at least 8 wk. Compared with wild-type mice, a proportion of T cell response in plt mice are shifted to the spleen, and prior splenectomy reduces the T cell response in draining lymph nodes. After immunization of plt mice, T cells and dendritic cells colocalize in the superficial cortex of lymph nodes and in splenic bridging channels, but not in T cell zones. These results demonstrate that plt mice mount robust T cell responses despite the failure of naive T cells and activated dendritic cells to enter the thymus dependent areas of secondary lymphoid organs.
Figures





References
-
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Lanzavecchia A., Sallusto F. Dynamics of T lymphocyte responsesintermediates, effectors, and memory cells. Science. 2000;290:92–97. - PubMed
-
- Cyster J.G. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. - PubMed
-
- Cyster J.G. Leukocyte migrationscent of the T zone. Curr. Biol. 2000;10:R30–R33. - PubMed
-
- Melchers F., Rolink A.G., Schaniel C. The role of chemokines in regulating cell migration during humoral immune responses. Cell. 1999;99:351–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

