The molybdenum cofactor biosynthetic protein Cnx1 complements molybdate-repairable mutants, transfers molybdenum to the metal binding pterin, and is associated with the cytoskeleton
- PMID: 11148290
- PMCID: PMC102230
- DOI: 10.1105/tpc.12.12.2455
The molybdenum cofactor biosynthetic protein Cnx1 complements molybdate-repairable mutants, transfers molybdenum to the metal binding pterin, and is associated with the cytoskeleton
Abstract
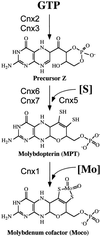
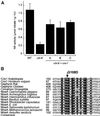
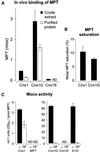
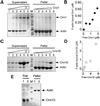
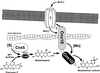
Molybdenum (Mo) plays an essential role in the active site of all eukaryotic Mo-containing enzymes. In plants, Mo enzymes are important for nitrate assimilation, phytohormone synthesis, and purine catabolism. Mo is bound to a unique metal binding pterin (molybdopterin [MPT]), thereby forming the active Mo cofactor (Moco), which is highly conserved in eukaryotes, eubacteria, and archaebacteria. Here, we describe the function of the two-domain protein Cnx1 from Arabidopsis in the final step of Moco biosynthesis. Cnx1 is constitutively expressed in all organs and in plants grown on different nitrogen sources. Mo-repairable cnxA mutants from Nicotiana plumbaginifolia accumulate MPT and show altered Cnx1 expression. Transformation of cnxA mutants and the corresponding Arabidopsis chl-6 mutant with cnx1 cDNA resulted in functional reconstitution of their Moco deficiency. We also identified a point mutation in the Cnx1 E domain of Arabidopsis chl-6 that causes the molybdate-repairable phenotype. Recombinant Cnx1 protein is capable of synthesizing Moco. The G domain binds and activates MPT, whereas the E domain is essential for activating Mo. In addition, Cnx1 binds to the cytoskeleton in the same way that its mammalian homolog gephyrin does in neuronal cells, which suggests a hypothetical model for anchoring the Moco-synthetic machinery by Cnx1 in plant cells.
Figures



Similar articles
-
Molybdenum co-factor biosynthesis: the Arabidopsis thaliana cDNA cnx1 encodes a multifunctional two-domain protein homologous to a mammalian neuroprotein, the insect protein Cinnamon and three Escherichia coli proteins.Plant J. 1995 Nov;8(5):751-62. doi: 10.1046/j.1365-313x.1995.08050751.x. Plant J. 1995. PMID: 8528286
-
Mutations in the molybdenum cofactor biosynthetic protein Cnx1G from Arabidopsis thaliana define functions for molybdopterin binding, molybdenum insertion, and molybdenum cofactor stabilization.Proc Natl Acad Sci U S A. 2000 Jun 6;97(12):6475-80. doi: 10.1073/pnas.110568497. Proc Natl Acad Sci U S A. 2000. PMID: 10823911 Free PMC article.
-
Crystal structures of human gephyrin and plant Cnx1 G domains: comparative analysis and functional implications.J Mol Biol. 2001 Sep 14;312(2):405-18. doi: 10.1006/jmbi.2001.4952. J Mol Biol. 2001. PMID: 11554796
-
Function of Molybdenum Insertases.Molecules. 2022 Aug 23;27(17):5372. doi: 10.3390/molecules27175372. Molecules. 2022. PMID: 36080140 Free PMC article. Review.
-
Molybdenum metabolism in the alga Chlamydomonas stands at the crossroad of those in Arabidopsis and humans.Metallomics. 2011 Jun;3(6):578-90. doi: 10.1039/c1mt00032b. Epub 2011 May 27. Metallomics. 2011. PMID: 21623427 Review.
Cited by
-
The Final Step in Molybdenum Cofactor Biosynthesis-A Historical View.Molecules. 2024 Sep 20;29(18):4458. doi: 10.3390/molecules29184458. Molecules. 2024. PMID: 39339452 Free PMC article. Review.
-
Insights into the Cnx1E catalyzed MPT-AMP hydrolysis.Biosci Rep. 2020 Jan 31;40(1):BSR20191806. doi: 10.1042/BSR20191806. Biosci Rep. 2020. PMID: 31860061 Free PMC article.
-
Crystal structures, dynamics and functional implications of molybdenum-cofactor biosynthesis protein MogA from two thermophilic organisms.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Jan 1;67(Pt 1):2-16. doi: 10.1107/S1744309110035037. Epub 2010 Dec 21. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011. PMID: 21206014 Free PMC article.
-
Copper-deficiency in Brassica napus induces copper remobilization, molybdenum accumulation and modification of the expression of chloroplastic proteins.PLoS One. 2014 Oct 15;9(10):e109889. doi: 10.1371/journal.pone.0109889. eCollection 2014. PLoS One. 2014. PMID: 25333918 Free PMC article.
-
Structural Evidence of Programmed Cell Death Induction by Tungsten in Root Tip Cells of Pisum sativum.Plants (Basel). 2019 Mar 11;8(3):62. doi: 10.3390/plants8030062. Plants (Basel). 2019. PMID: 30862127 Free PMC article.
References
-
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C.R. Acad. Sci. Paris Sci. Vie 316 1194–1199.
-
- Braaksma, F.J., and Feenstra, W.J. (1982). Isolation and characterization of nitrate reductase–deficient mutants of Arabidopsis thaliana. Theor. Appl. Genet. 64 83–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

