Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity
- PMID: 11148293
- PMCID: PMC102233
- DOI: 10.1105/tpc.12.12.2499
Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity
Abstract
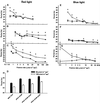
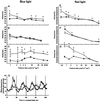
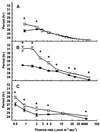
The circadian clock is entrained to the daily cycle of day and night by light signals at dawn and dusk. Plants make use of both the phytochrome (phy) and cryptochrome (cry) families of photoreceptors in gathering information about the light environment for setting the clock. We demonstrate that the phytochromes phyA, phyB, phyD, and phyE act as photoreceptors in red light input to the clock and that phyA and the cryptochromes cry1 and cry2 act as photoreceptors in blue light input. phyA and phyB act additively in red light input to the clock, whereas cry1 and cry2 act redundantly in blue light input. In addition to the action of cry1 as a photoreceptor that mediates blue light input into the clock, we demonstrate a requirement of cry1 for phyA signaling to the clock in both red and blue light. Importantly, Arabidopsis cry1 cry2 double mutants still show robust rhythmicity, indicating that cryptochromes do not form a part of the central circadian oscillator in plants as they do in mammals.
Figures
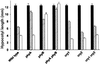
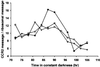
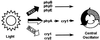
References
-
- Ahmad, M., and Cashmore, A.R. (1993). HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366 162–166. - PubMed
-
- Ahmad, M., Jarillo, J.A., Smirnova, O., and Cashmore, A.R. (1998). The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Mol. Cell 1 939–948. - PubMed
-
- Aschoff, J. (1979). Circadian rhythms: Influences of internal and external factors on the period measured in constant conditions. Z. Tierpsychol. 49 225–249. - PubMed
-
- Cashmore, A., Jarillo, J.A., Wu, Y.J., and Liu, D. (1999). Cryptochromes: Blue light receptors for plants and animals. Science 284 760–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

