Membrane recruitment of Aut7p in the autophagy and cytoplasm to vacuole targeting pathways requires Aut1p, Aut2p, and the autophagy conjugation complex
- PMID: 11149920
- PMCID: PMC2193654
- DOI: 10.1083/jcb.152.1.51
Membrane recruitment of Aut7p in the autophagy and cytoplasm to vacuole targeting pathways requires Aut1p, Aut2p, and the autophagy conjugation complex
Abstract
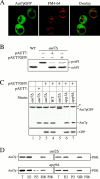
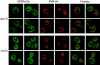
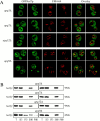
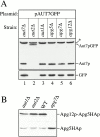
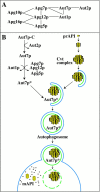
Autophagy is a degradative pathway by which cells sequester nonessential, bulk cytosol into double-membrane vesicles (autophagosomes) and deliver them to the vacuole for recycling. Using this strategy, eukaryotic cells survive periods of nutritional starvation. Under nutrient-rich conditions, autophagy machinery is required for the delivery of a resident vacuolar hydrolase, aminopeptidase I, by the cytoplasm to vacuole targeting (Cvt) pathway. In both pathways, the vesicle formation process requires the function of the starvation-induced Aut7 protein, which is recruited from the cytosol to the forming Cvt vesicles and autophagosomes. The membrane binding of Aut7p represents an early step in vesicle formation. In this study, we identify several requirements for Aut7p membrane association. After synthesis in the cytosol, Aut7p is proteolytically cleaved in an Aut2p-dependent manner. While this novel processing event is essential for Aut7p membrane binding, Aut7p must undergo additional physical interactions with Aut1p and the autophagy (Apg) conjugation complex before recruitment to the membrane. Lack of these interactions results in a cytosolic distribution of Aut7p rather than localization to forming Cvt vesicles and autophagosomes. This study assigns a functional role for the Apg conjugation system as a mediator of Aut7p membrane recruitment. Further, we demonstrate that Aut1p, which physically interacts with components of the Apg conjugation complex and Aut7p, constitutes an additional factor required for Aut7p membrane recruitment. These findings define a series of steps that results in the modification of Aut7p and its subsequent binding to the sequestering transport vesicles of the autophagy and cytoplasm to vacuole targeting pathways.
Figures





References
-
- Gerhardt B., Kordas T.J., Thompson C.M., Patel P., Vida T. The vesicle transport protein Vps33p is an ATP-binding protein that localizes to the cytosol in an energy-dependent manner. J. Biol. Chem. 1998;273:15818–15829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

