Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding
- PMID: 11149925
- PMCID: PMC2193662
- DOI: 10.1083/jcb.152.1.111
Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding
Abstract
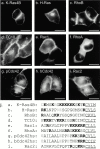
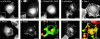
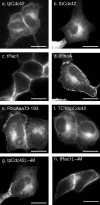
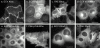
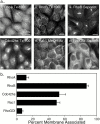
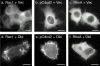
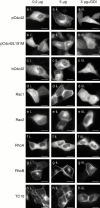
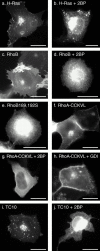
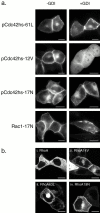
Determinants of membrane targeting of Rho proteins were investigated in live cells with green fluorescent fusion proteins expressed with or without Rho-guanine nucleotide dissociation inhibitor (GDI)alpha. The hypervariable region determined to which membrane compartment each protein was targeted. Targeting was regulated by binding to RhoGDI alpha in the case of RhoA, Rac1, Rac2, and Cdc42hs but not RhoB or TC10. Although RhoB localized to the plasma membrane (PM), Golgi, and motile peri-Golgi vesicles, TC10 localized to PMs and endosomes. Inhibition of palmitoylation mislocalized H-Ras, RhoB, and TC10 to the endoplasmic reticulum. Although overexpressed Cdc42hs and Rac2 were observed predominantly on endomembrane, Rac1 was predominantly at the PM. RhoA was cytosolic even when expressed at levels in vast excess of RhoGDI alpha. Oncogenic Dbl stimulated translocation of green fluorescent protein (GFP)-Rac1, GFP-Cdc42hs, and GFP-RhoA to lamellipodia. RhoGDI binding to GFP-Cdc42hs was not affected by substituting farnesylation for geranylgeranylation. A palmitoylation site inserted into RhoA blocked RhoGDI alpha binding. Mutations that render RhoA, Cdc42hs, or Rac1, either constitutively active or dominant negative abrogated binding to RhoGDI alpha and redirected expression to both PMs and internal membranes. Thus, despite the common essential feature of the CAAX (prenylation, AAX tripeptide proteolysis, and carboxyl methylation) motif, the subcellular localizations of Rho GTPases, like their functions, are diverse and dynamic.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

