The Telomerase/vault-associated protein TEP1 is required for vault RNA stability and its association with the vault particle
- PMID: 11149928
- PMCID: PMC2193651
- DOI: 10.1083/jcb.152.1.157
The Telomerase/vault-associated protein TEP1 is required for vault RNA stability and its association with the vault particle
Abstract
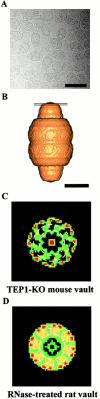
Vaults and telomerase are ribonucleoprotein (RNP) particles that share a common protein subunit, TEP1. Although its role in either complex has not yet been defined, TEP1 has been shown to interact with the mouse telomerase RNA and with several of the human vault RNAs in a yeast three-hybrid assay. An mTep1(-/-) mouse was previously generated which resulted in no apparent change in telomere length or telomerase activity in six generations of mTep1-deficient mice. Here we show that the levels of the telomerase RNA and its association with the telomerase RNP are also unaffected in mTep1(-/-) mice. Although vaults purified from the livers of mTep1(-/-) mice appear structurally intact by both negative stain and cryoelectron microscopy, three-dimensional reconstruction of the mTep1(-/-) vault revealed less density in the cap than previously observed for the intact rat vault. Furthermore, the absence of TEP1 completely disrupted the stable association of the vault RNA with the purified vault particle and also resulted in a decrease in the levels and stability of the vault RNA. Therefore, we have uncovered a novel role for TEP1 in vivo as an integral vault protein important for the stabilization and recruitment of the vault RNA to the vault particle.
Figures









References
-
- Adrian M., Dubochet J., Lepault J., McDowall A.W. Cryo-electron microscopy of viruses. Nature. 1984;308:32–36. - PubMed
-
- Beattie T.L., Zhou W., Robinson M.O., Harrington L. Reconstitution of human telomerase activity in vitro . Curr. Biol. 1998;8:177–180. - PubMed
-
- Blasco M.A., Funk W., Villeponteau B., Greider C.W. Functional characterization and developmental regulation of mouse telomerase RNA. Science. 1995;269:1267–1270. - PubMed
-
- Böttcher B., Wynne S.A., Crowther R.A. Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature. 1997;386:88–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

