Cbl associates with Pyk2 and Src to regulate Src kinase activity, alpha(v)beta(3) integrin-mediated signaling, cell adhesion, and osteoclast motility
- PMID: 11149930
- PMCID: PMC2193648
- DOI: 10.1083/jcb.152.1.181
Cbl associates with Pyk2 and Src to regulate Src kinase activity, alpha(v)beta(3) integrin-mediated signaling, cell adhesion, and osteoclast motility
Abstract
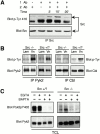
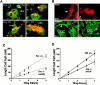
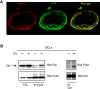
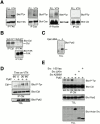
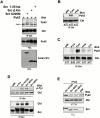
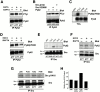
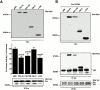
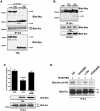
The signaling events downstream of integrins that regulate cell attachment and motility are only partially understood. Using osteoclasts and transfected 293 cells, we find that a molecular complex comprising Src, Pyk2, and Cbl functions to regulate cell adhesion and motility. The activation of integrin alpha(v)beta(3) induces the [Ca(2+)](i)-dependent phosphorylation of Pyk2 Y402, its association with Src SH2, Src activation, and the Src SH3-dependent recruitment and phosphorylation of c-Cbl. Furthermore, the PTB domain of Cbl is shown to bind to phosphorylated Tyr-416 in the activation loop of Src, the autophosphorylation site of Src, inhibiting Src kinase activity and integrin-mediated adhesion. Finally, we show that deletion of c Src or c-Cbl leads to a decrease in osteoclast migration. Thus, binding of alpha(v)beta(3) integrin induces the formation of a Pyk2/Src/Cbl complex in which Cbl is a key regulator of Src kinase activity and of cell adhesion and migration. These findings may explain the osteopetrotic phenotype in the Src(-/-) mice.
Figures
References
-
- Barber D.L., Mason J.M., Fukazawa T., Reedquist K.A., Druker B.J., Band H., D'Andrea A.D. Erythropoietin and interleukin-3 activate tyrosine phosphorylation of CBL and association with CRK adaptor proteins. Blood. 1997;89:3166–3174. - PubMed
-
- Bartkiewicz M., Houghton A., Baron R. Leucine zipper-mediated homodimerization of the adaptor protein c-Cbla role in c-Cbl's tyrosine phosphorylation and its association with epidermal growth factor receptor. J. Biol. Chem. 1999;274:30887–30895. - PubMed
-
- Bonita D.P., Miyake S., Lupher M.L., Jr., Langdon W.Y., Band H. Phosphotyrosine binding domain-dependent upregulation of the platelet-derived growth factor receptor α signaling cascade by transforming mutants of Cblimplications for Cbl's function and oncogenicity. Mol. Cell. Biol. 1997;17:4597–4610. - PMC - PubMed
-
- Broome M.A., Galisteo M.L., Schlessinger J., Courtnedge S.A. The protooncogene c-Cbl is a negative regulator of DNA synthesis initiated by both receptor and cytoplasmic tyrosine kinases. Oncogene. 1999;18:2908–2912. - PubMed
-
- Chen W.T. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J. Exp. Zool. 1989;251:167–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

