Nerve growth factor, but not epidermal growth factor, increases Fra-2 expression and alters Fra-2/JunD binding to AP-1 and CREB binding elements in pheochromocytoma (PC12) cells
- PMID: 11150315
- PMCID: PMC6762456
- DOI: 10.1523/JNEUROSCI.21-01-00018.2001
Nerve growth factor, but not epidermal growth factor, increases Fra-2 expression and alters Fra-2/JunD binding to AP-1 and CREB binding elements in pheochromocytoma (PC12) cells
Abstract
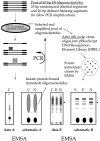
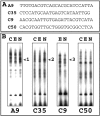
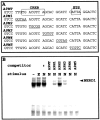
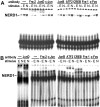
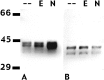
In pheochromocytoma (PC12) cells nerve growth factor (NGF) and epidermal growth factor (EGF) activate similar receptor tyrosine kinase signaling pathways but evoke strikingly different biological outcomes: NGF induces differentiation and EGF acts as a mitogen. A novel approach was developed for identifying transcription factor activities associated with NGF-activated, but not EGF-activated, signaling, using random oligonucleotide clones from a DNA recognition library to isolate specific DNA binding proteins from PC12 nuclear extracts. A protein complex from NGF-treated, but not EGF-treated, cells was identified that exhibits increased mobility and DNA binding activity in gel mobility shift assays. The binding complex was identified in supershift assays as Fra-2/JunD. The clones used as probes contain either AP-1 or cAMP response element binding (CREB) recognition elements. Time course experiments revealed further differences in NGF and EGF signaling in PC12 cells. NGF elicits a more delayed and sustained ERK phosphorylation than EGF, consistent with previous reports. Both growth factors transiently induce c-fos, but NGF evokes a greater response than EGF. NGF specifically increases Fra-1 and Fra-2 levels at 4 and 24 hr. The latter is represented in Western blots by bands in the 40-46 kDa range. NGF, but not EGF, enhances the upper bands, corresponding to phosphorylated Fra-2. These findings suggest that prolonged alterations in Fra-2 and subsequent increases in Fra-2/JunD binding to AP-1 and CREB response elements common among many gene promoters could serve to trigger broadly an NGF-specific program of gene expression.
Figures


Similar articles
-
Nerve growth factor- and epidermal growth factor-regulated gene transcription in PC12 pheochromocytoma and INS-1 insulinoma cells.Eur J Cell Biol. 2000 Dec;79(12):924-35. doi: 10.1078/0171-9335-00126. Eur J Cell Biol. 2000. PMID: 11152283
-
Global expression analysis identified a preferentially nerve growth factor-induced transcriptional program regulated by sustained mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) and AP-1 protein activation during PC12 cell differentiation.J Biol Chem. 2011 Dec 30;286(52):45131-45. doi: 10.1074/jbc.M111.274076. Epub 2011 Nov 7. J Biol Chem. 2011. PMID: 22065583 Free PMC article.
-
Inhibition of PC-12 cell differentiation by the immediate early gene fra-1.Oncogene. 1990 Dec;5(12):1755-60. Oncogene. 1990. PMID: 2178237
-
Signaling pathways for PC12 cell differentiation: making the right connections.Science. 2002 May 31;296(5573):1648-9. doi: 10.1126/science.1071552. Science. 2002. PMID: 12040181 Review.
-
Role of Fra-2 in cancer.Cell Death Differ. 2024 Feb;31(2):136-149. doi: 10.1038/s41418-023-01248-4. Epub 2023 Dec 16. Cell Death Differ. 2024. PMID: 38104183 Free PMC article. Review.
Cited by
-
The transcriptional response to hypoxic insult controlled by FRA-2.Gene Expr. 2005;12(2):61-7. doi: 10.3727/000000005783992160. Gene Expr. 2005. PMID: 15892448 Free PMC article.
-
Progressive accumulation of activated ERK2 within highly stable ORF45-containing nuclear complexes promotes lytic gammaherpesvirus infection.PLoS Pathog. 2014 Apr 10;10(4):e1004066. doi: 10.1371/journal.ppat.1004066. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24722398 Free PMC article.
-
CaMKII-Mediated CREB Phosphorylation Is Involved in Ca2+-Induced BDNF mRNA Transcription and Neurite Outgrowth Promoted by Electrical Stimulation.PLoS One. 2016 Sep 9;11(9):e0162784. doi: 10.1371/journal.pone.0162784. eCollection 2016. PLoS One. 2016. PMID: 27611779 Free PMC article.
-
Critical role for Kalirin in nerve growth factor signaling through TrkA.Mol Cell Biol. 2005 Jun;25(12):5106-18. doi: 10.1128/MCB.25.12.5106-5118.2005. Mol Cell Biol. 2005. PMID: 15923627 Free PMC article.
-
BMP enhances transcriptional responses to NGF during PC12 cell differentiation.Neurochem Res. 2005 Jun-Jul;30(6-7):753-65. doi: 10.1007/s11064-005-6868-6. Neurochem Res. 2005. PMID: 16187211
References
-
- Abmayr SM, Workman JL. Preparation of nuclear and cytoplasmic extracts from mammalian cells. In: Ausubel FM, Brent R, Kingston RE, editors. Current protocols in molecular biology. Breene and Wiley-Interscience; New York: 1989. pp. 12.2.1–12.2.10. - PubMed
-
- Alheim K, McDowell TL, Symons JA, Duff GW, Bartfai T. An AP-1 site is involved in the NGF induction of IL-1α in PC12 cells. Neurochem Int. 1996;29:487–496. - PubMed
-
- Angel P, Karin M. The role of Jun, Fos, and the AP-1 complex in cell proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. - PubMed
-
- Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahmsdorf HJ, Jonat C, Herrlich P, Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–739. - PubMed
-
- Bejanin S, Habert E, Berrard S, Dumas J-B, Edwards M, Loeffler J-P, Mallet J. Promoter elements of the rat choline acetyltransferase gene allowing nerve growth factor inducibility in transfected primary cultured cells. J Neurochem. 1992;58:1580–1583. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
