Presynaptic kainate receptors regulate spinal sensory transmission
- PMID: 11150320
- PMCID: PMC6762455
- DOI: 10.1523/JNEUROSCI.21-01-00059.2001
Presynaptic kainate receptors regulate spinal sensory transmission
Abstract
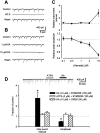
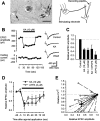
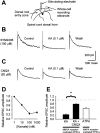
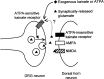
Small diameter dorsal root ganglion (DRG) neurons, which include cells that transmit nociceptive information into the spinal cord, are known to express functional kainate receptors. It is well established that exposure to kainate will depolarize C-fiber afferents arising from these cells. Although the role of kainate receptors on sensory afferents is unknown, it has been hypothesized that presynaptic kainate receptors may regulate glutamate release in the spinal cord. Here we show that kainate, applied at low micromolar concentrations in the presence of the AMPA-selective antagonist (RS)-4-(4-aminophenyl)-1, 2-dihydro-1-methyl-2-propyl-carbamoyl-6,7-methylenedioxyphthalazine++ +, suppressed spontaneous NMDA receptor-mediated EPSCs in cultures of spinal dorsal horn neurons. In addition, kainate suppressed EPSCs in dorsal horn neurons evoked by stimulation of synaptically coupled DRG cells in DRG-dorsal horn neuron cocultures. Interestingly, although the glutamate receptor subunit 5-selective kainate receptor agonist (RS)-2-alpha-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid (ATPA) (2 micrometer) was able to suppress DRG-dorsal horn synaptic transmission to a similar extent as kainate (10 micrometer), it had no effect on excitatory transmission between dorsal horn neurons. Agonist applications revealed a striking difference between kainate receptors expressed by DRG and dorsal horn neurons. Whereas DRG cell kainate receptors were sensitive to both kainate and ATPA, most dorsal horn neurons responded only to kainate. Finally, in recordings from dorsal horn neurons in spinal slices, kainate and ATPA were able to suppress NMDA and AMPA receptor-mediated EPSCs evoked by dorsal root fiber stimulation. Together, these data suggest that kainate receptor agonists, acting at a presynaptic locus, can reduce glutamate release from primary afferent sensory synapses.
Figures


Similar articles
-
Functional similarities and differences of AMPA and kainate receptors expressed by cultured rat sensory neurons.Neuroscience. 2004;129(1):35-48. doi: 10.1016/j.neuroscience.2004.07.015. Neuroscience. 2004. PMID: 15489026
-
Kainate receptor subunits underlying presynaptic regulation of transmitter release in the dorsal horn.J Neurosci. 2002 Sep 15;22(18):8010-7. doi: 10.1523/JNEUROSCI.22-18-08010.2002. J Neurosci. 2002. PMID: 12223554 Free PMC article.
-
Cholinergic modulation of primary afferent glutamatergic transmission in rat medullary dorsal horn neurons.Neuropharmacology. 2013 Dec;75:295-303. doi: 10.1016/j.neuropharm.2013.07.030. Epub 2013 Aug 13. Neuropharmacology. 2013. PMID: 23954675
-
Modulation of sensory input to the spinal cord by presynaptic ionotropic glutamate receptors.Arch Ital Biol. 2005 May;143(2):103-12. Arch Ital Biol. 2005. PMID: 16106991 Review.
-
Role of kainate receptors in nociception.Brain Res Brain Res Rev. 2002 Oct;40(1-3):215-22. doi: 10.1016/s0165-0173(02)00203-5. Brain Res Brain Res Rev. 2002. PMID: 12589919 Review.
Cited by
-
Functional diversity and developmental changes in rat neuronal kainate receptors.J Physiol. 2001 Apr 15;532(Pt 2):411-21. doi: 10.1111/j.1469-7793.2001.0411f.x. J Physiol. 2001. PMID: 11306660 Free PMC article.
-
SCRAPPER Selectively Contributes to Spontaneous Release and Presynaptic Long-Term Potentiation in the Anterior Cingulate Cortex.J Neurosci. 2017 Apr 5;37(14):3887-3895. doi: 10.1523/JNEUROSCI.0023-16.2017. Epub 2017 Mar 14. J Neurosci. 2017. PMID: 28292828 Free PMC article.
-
Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn.J Neurosci. 2004 Mar 17;24(11):2774-81. doi: 10.1523/JNEUROSCI.4637-03.2004. J Neurosci. 2004. PMID: 15028770 Free PMC article.
-
Presynaptic regulation of the inhibitory transmission by GluR5-containing kainate receptors in spinal substantia gelatinosa.Mol Pain. 2006 Sep 1;2:29. doi: 10.1186/1744-8069-2-29. Mol Pain. 2006. PMID: 16948848 Free PMC article.
-
Activation of iGluR5 kainate receptors inhibits neurogenic dural vasodilatation in an animal model of trigeminovascular activation.Br J Pharmacol. 2009 Jun;157(3):464-73. doi: 10.1111/j.1476-5381.2009.00142.x. Epub 2009 Mar 20. Br J Pharmacol. 2009. PMID: 19309356 Free PMC article.
References
-
- Ault B, Hildebrand LM. Activation of nociceptive reflexes by peripheral kainate receptors. J Pharmacol Exp Ther. 1993;265:927–932. - PubMed
-
- Bekkers JM, Stevens CF. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990;346:724–729. - PubMed
-
- Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
