Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons
- PMID: 11150325
- PMCID: PMC6762438
- DOI: 10.1523/JNEUROSCI.21-01-00098.2001
Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons
Abstract
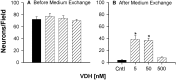
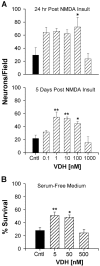
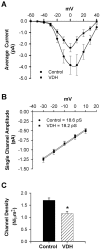
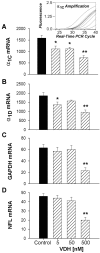
Although vitamin D hormone (VDH; 1,25-dihydroxyvitamin D(3)), the active metabolite of vitamin D, is the major Ca(2+)-regulatory steroid hormone in the periphery, it is not known whether it also modulates Ca(2+) homeostasis in brain neurons. Recently, chronic treatment with VDH was reported to protect brain neurons in both aging and animal models of stroke. However, it is unclear whether those actions were attributable to direct effects on brain cells or indirect effects mediated via peripheral pathways. VDH modulates L-type voltage-sensitive Ca(2+) channels (L-VSCCs) in peripheral tissues, and an increase in L-VSCCs appears linked to both brain aging and neuronal vulnerability. Therefore, we tested the hypothesis that VDH has direct neuroprotective actions and, in parallel, targets L-VSCCs in hippocampal neurons. Primary rat hippocampal cultures, treated for several days with VDH, exhibited a U-shaped concentration-response curve for neuroprotection against excitotoxic insults: lower concentrations of VDH (1-100 nm) were protective, but higher, nonphysiological concentrations (500-1000 nm) were not. Parallel studies using patch-clamp techniques found a similar U-shaped curve in which L-VSCC current was reduced at lower VDH concentrations and increased at higher (500 nm) concentrations. Real-time PCR studies demonstrated that VDH monotonically downregulated mRNA expression for the alpha(1C) and alpha(1D) pore-forming subunits of L-VSCCs. However, 500 nm VDH also nonspecifically reduced a range of other mRNA species. Thus, these studies provide the first evidence of (1) direct neuroprotective actions of VDH at relatively low concentrations, and (2) selective downregulation of L-VSCC expression in brain neurons at the same, lower concentrations.
Figures



References
-
- Alexianu ME, Robbins E, Carswell S, Appel SH. 1α,25 Dihydroxyvitamin D3-dependent up-regulation of calcium binding proteins in motoneurons cells. J Neurosci Res. 1998;51:58–66. - PubMed
-
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. - PubMed
-
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–425. - PubMed
-
- Bean BP. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. - PubMed
-
- Bito H, Deisseroth K, Tsien RW. Ca2+-dependent regulation in neuronal gene expression. Curr Opin Neurobiol. 1997;7:419–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
