Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens
- PMID: 11150326
- PMCID: PMC6762427
- DOI: 10.1523/JNEUROSCI.21-01-00109.2001
Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens
Abstract
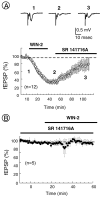
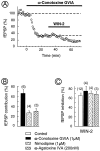
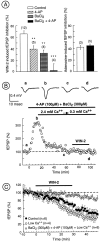
Despite the role of excitatory transmission to the nucleus accumbens (NAc) in the actions of most drugs of abuse, the presence and functions of cannabinoid receptors (CB1) on the glutamatergic cortical afferents to the NAc have never been explored. Here, immunohistochemistry has been used to show the localization of CB1 receptors on axonal terminals making contacts with the NAc GABAergic neurons. Electrophysiological techniques in the NAc slice preparation revealed that cannabimimetics [WIN 55,212,2 (WIN-2) and CP55940] strongly inhibit stimulus-evoked glutamate-mediated transmission. The inhibitory actions of WIN-2 were dose-dependent (EC(50) of 293 +/- 13 nm) and reversed by the selective CB1 antagonist SR 141716A. In agreement with a presynaptic localization of CB1 receptors, WIN-2 increased paired-pulse facilitation, decreased miniature EPSC (mEPSC) frequency, and had no effect on the mEPSCs amplitude. Perfusion with the adenylate cyclase activator forskolin enhanced glutamatergic transmission but did not alter presynaptic CB1 actions, suggesting that cannabinoids inhibit glutamate release independently from the cAMP-PKA cascade. CB1 did not reduce evoked transmitter release by inhibiting presynaptic voltage-dependent Ca(2+) currents through N-, L-, or P/Q-type Ca(2+) channels, because CB1 inhibition persisted in the presence of omega-Conotoxin-GVIA, nimodipine, or omega-Agatoxin-IVA. The K(+) channel blockers 4-aminopyridine (100 micrometer) and BaCl(2) (300 micrometer) each reduced by 40-50% the inhibitory actions of WIN-2, and their effects were additive. These data suggest that CB1 receptors are located on the cortical afferents to the nucleus and can reduce glutamate synaptic transmission within the NAc by modulating K(+) channels activity.
Figures




Similar articles
-
Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons.J Physiol. 2001 May 1;532(Pt 3):731-48. doi: 10.1111/j.1469-7793.2001.0731e.x. J Physiol. 2001. PMID: 11313442 Free PMC article.
-
Role of p/q-Ca2+ channels in metabotropic glutamate receptor 2/3-dependent presynaptic long-term depression at nucleus accumbens synapses.J Neurosci. 2002 Jun 1;22(11):4346-56. doi: 10.1523/JNEUROSCI.22-11-04346.2002. J Neurosci. 2002. PMID: 12040040 Free PMC article.
-
Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids.J Neurophysiol. 2001 Jan;85(1):72-83. doi: 10.1152/jn.2001.85.1.72. J Neurophysiol. 2001. PMID: 11152707
-
Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition.Chem Phys Lipids. 2002 Dec 31;121(1-2):73-82. doi: 10.1016/s0009-3084(02)00149-4. Chem Phys Lipids. 2002. PMID: 12505692 Review.
-
Effects of cannabinoids on neurotransmission.Handb Exp Pharmacol. 2005;(168):327-65. doi: 10.1007/3-540-26573-2_11. Handb Exp Pharmacol. 2005. PMID: 16596780 Review.
Cited by
-
Marijuana and cannabinoid regulation of brain reward circuits.Br J Pharmacol. 2004 Sep;143(2):227-34. doi: 10.1038/sj.bjp.0705931. Epub 2004 Aug 16. Br J Pharmacol. 2004. PMID: 15313883 Free PMC article. Review.
-
Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum.J Neurosci. 2005 Nov 9;25(45):10537-45. doi: 10.1523/JNEUROSCI.2959-05.2005. J Neurosci. 2005. PMID: 16280591 Free PMC article.
-
Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex.PLoS One. 2007 Aug 8;2(8):e709. doi: 10.1371/journal.pone.0000709. PLoS One. 2007. PMID: 17684555 Free PMC article.
-
Neuronal GHS-R Differentially Modulates Feeding Patterns under Normal and Obesogenic Conditions.Biomolecules. 2022 Feb 11;12(2):293. doi: 10.3390/biom12020293. Biomolecules. 2022. PMID: 35204795 Free PMC article.
-
A Model of Δ9-Tetrahydrocannabinol Self-administration and Reinstatement That Alters Synaptic Plasticity in Nucleus Accumbens.Biol Psychiatry. 2018 Oct 15;84(8):601-610. doi: 10.1016/j.biopsych.2018.04.016. Epub 2018 May 3. Biol Psychiatry. 2018. PMID: 29861097 Free PMC article.
References
-
- Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol. 2000;83:3287–3293. - PubMed
-
- Chan PK, Yung WH. Occlusion of the presynaptic action of cannabinoids in rat substantia nigra pars reticulata by cadmium. Neurosci Lett. 1998;249:57–60. - PubMed
-
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega- Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann NY Acad Sci. 1999;868:233–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
