The contribution of dendritic Kv3 K+ channels to burst threshold in a sensory neuron
- PMID: 11150328
- PMCID: PMC6762436
- DOI: 10.1523/JNEUROSCI.21-01-00125.2001
The contribution of dendritic Kv3 K+ channels to burst threshold in a sensory neuron
Abstract
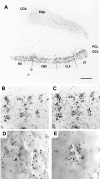
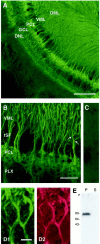
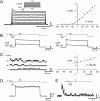
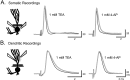
Voltage-gated ion channels localized to dendritic membranes can shape signal processing in central neurons. This study describes the distribution and functional role of a high voltage-activating K(+) channel in the electrosensory lobe (ELL) of an apteronotid weakly electric fish. We identify a homolog of the Kv3.3 K(+) channel, AptKv3.3, that exhibits a high density of mRNA expression and immunolabel that is distributed over the entire soma-dendritic axis of ELL pyramidal cells. The kinetics and pharmacology of native K(+) channels recorded in pyramidal cell somata and apical dendrites match those of AptKv3.3 channels expressed in a heterologous expression system. The functional role of AptKv3.3 channels was assessed using focal drug ejections in somatic and dendritic regions of an in vitro slice preparation. Local blockade of AptKv3.3 channels slows the repolarization of spikes in pyramidal cell somata as well as spikes backpropagating into apical dendrites. The resulting increase in dendritic spike duration lowers the threshold for a gamma-frequency burst discharge that is driven by inward current associated with backpropagating dendritic spikes. Thus, dendritic AptKv3.3 K(+) channels influence the threshold for a form of burst discharge that has an established role in feature extraction of sensory input.
Figures



Similar articles
-
Conditional spike backpropagation generates burst discharge in a sensory neuron.J Neurophysiol. 2000 Sep;84(3):1519-30. doi: 10.1152/jn.2000.84.3.1519. J Neurophysiol. 2000. PMID: 10980024
-
A prominent soma-dendritic distribution of Kv3.3 K+ channels in electrosensory and cerebellar neurons.J Comp Neurol. 2001 Dec 17;441(3):234-47. doi: 10.1002/cne.1409. J Comp Neurol. 2001. PMID: 11745647
-
TTX-sensitive dendritic sodium channels underlie oscillatory discharge in a vertebrate sensory neuron.J Neurosci. 1994 Nov;14(11 Pt 1):6453-71. doi: 10.1523/JNEUROSCI.14-11-06453.1994. J Neurosci. 1994. PMID: 7965050 Free PMC article.
-
Oscillatory burst discharge generated through conditional backpropagation of dendritic spikes.J Physiol Paris. 2002 Sep-Dec;96(5-6):517-30. doi: 10.1016/S0928-4257(03)00007-X. J Physiol Paris. 2002. PMID: 14692499 Review.
-
Distribution and function of potassium channels in the electrosensory lateral line lobe of weakly electric apteronotid fish.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006 Jun;192(6):637-48. doi: 10.1007/s00359-006-0103-z. Epub 2006 Jan 20. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006. PMID: 16425062 Review.
Cited by
-
Releasing the peri-neuronal net to patch-clamp neurons in adult CNS.Pflugers Arch. 2004 May;448(2):248-58. doi: 10.1007/s00424-004-1246-9. Epub 2004 Feb 17. Pflugers Arch. 2004. PMID: 14985983
-
A dynamic dendritic refractory period regulates burst discharge in the electrosensory lobe of weakly electric fish.J Neurosci. 2003 Feb 15;23(4):1524-34. doi: 10.1523/JNEUROSCI.23-04-01524.2003. J Neurosci. 2003. PMID: 12598641 Free PMC article.
-
A C-terminal domain directs Kv3.3 channels to dendrites.J Neurosci. 2005 Dec 14;25(50):11531-41. doi: 10.1523/JNEUROSCI.3672-05.2005. J Neurosci. 2005. PMID: 16354911 Free PMC article.
-
Ionic and neuromodulatory regulation of burst discharge controls frequency tuning.J Physiol Paris. 2008 Jul-Nov;102(4-6):195-208. doi: 10.1016/j.jphysparis.2008.10.019. Epub 2008 Oct 18. J Physiol Paris. 2008. PMID: 18992813 Free PMC article. Review.
-
Ghostbursting: a novel neuronal burst mechanism.J Comput Neurosci. 2002 Jan-Feb;12(1):5-25. doi: 10.1023/a:1014921628797. J Comput Neurosci. 2002. PMID: 11932557
References
-
- Berman NJ, Maler L. Neural architecture of the electrosensory lateral line lobe: adaptations for coincidence detection, a sensory searchlight and frequency-dependent adaptive filtering. J Exp Biol. 1999;202:1243–1253. - PubMed
-
- Bottai D, Dunn RJ, Ellis W, Maler L. N-methyl-d-aspartate receptor 1 mRNA distribution in the central nervous system of the weakly electric fish Apteronotus leptorhychus. J Comp Neurol. 1997;389:65–80. - PubMed
-
- Chandy KG, Gutman GA. Voltage gated channels. In: North RA, editor. Handbook of receptors and channels: ligand-gated and voltage-gated ion channels. CRC; Boca Raton, FL: 1995. pp. 1–71.
-
- Chen WR, Midtgaard J, Shepherd GM. Forward and backward propagation of dendritic impulses and their synaptic control in mitral cells. Science. 1997;278:463–467. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
