Presynaptic role of cGMP-dependent protein kinase during long-lasting potentiation
- PMID: 11150330
- PMCID: PMC6762426
- DOI: 10.1523/JNEUROSCI.21-01-00143.2001
Presynaptic role of cGMP-dependent protein kinase during long-lasting potentiation
Abstract
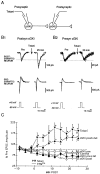
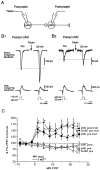
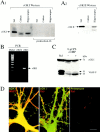
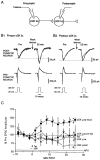
Previous research has suggested that cGMP-dependent protein kinases (cGKs) may play a role in long-term potentiation in hippocampus, but their site of action has been unknown. We examined this question at synapses between pairs of hippocampal neurons in dissociated cell culture. Injection of a specific peptide inhibitor of cGK into the presynaptic but not the postsynaptic neuron blocked long-lasting potentiation induced by tetanic stimulation of the presynaptic neuron. As controls, injection of a scrambled peptide or a peptide inhibitor of cAMP-dependent protein kinase into either neuron did not block potentiation. Conversely, injection of the alpha isozyme of cGK type I into the presynaptic but not the postsynaptic neuron produced activity-dependent potentiation that did not require NMDA receptor activation. Evidence from Western blots, reverse transcription-PCR, activity assays, and immunocytochemistry indicates that endogenous cGK type I is present in the neurons, including presynaptic terminals. These results support the idea that cGK plays an important presynaptic role during the induction of long-lasting potentiation in hippocampal neurons.
Figures
Similar articles
-
Long-term potentiation in hippocampus involves sequential activation of soluble guanylate cyclase, cGMP-dependent protein kinase, and cGMP-degrading phosphodiesterase.J Neurosci. 2002 Dec 1;22(23):10116-22. doi: 10.1523/JNEUROSCI.22-23-10116.2002. J Neurosci. 2002. PMID: 12451112 Free PMC article.
-
Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins.Neuron. 2005 Feb 3;45(3):389-403. doi: 10.1016/j.neuron.2005.01.011. Neuron. 2005. PMID: 15694326
-
Activity-dependent long-term enhancement of transmitter release by presynaptic 3',5'-cyclic GMP in cultured hippocampal neurons.Nature. 1995 Jul 6;376(6535):74-80. doi: 10.1038/376074a0. Nature. 1995. PMID: 7596438
-
Nitric oxide and carbon monoxide as possible retrograde messengers in hippocampal long-term potentiation.J Neurobiol. 1994 Jun;25(6):652-65. doi: 10.1002/neu.480250607. J Neurobiol. 1994. PMID: 8071665 Review.
-
Sequential activation of soluble guanylate cyclase, protein kinase G and cGMP-degrading phosphodiesterase is necessary for proper induction of long-term potentiation in CA1 of hippocampus. Alterations in hyperammonemia.Neurochem Int. 2004 Nov;45(6):895-901. doi: 10.1016/j.neuint.2004.03.020. Neurochem Int. 2004. PMID: 15312984 Review.
Cited by
-
Long-term potentiation in hippocampus involves sequential activation of soluble guanylate cyclase, cGMP-dependent protein kinase, and cGMP-degrading phosphodiesterase.J Neurosci. 2002 Dec 1;22(23):10116-22. doi: 10.1523/JNEUROSCI.22-23-10116.2002. J Neurosci. 2002. PMID: 12451112 Free PMC article.
-
Impairments in fear conditioning in mice lacking the nNOS gene.Learn Mem. 2009 May 23;16(6):371-8. doi: 10.1101/lm.1329209. Print 2009 Jun. Learn Mem. 2009. PMID: 19470653 Free PMC article.
-
The Phosphodiesterase 9 Inhibitor PF-04449613 Promotes Dendritic Spine Formation and Performance Improvement after Motor Learning.Dev Neurobiol. 2018 Sep;78(9):859-872. doi: 10.1002/dneu.22623. Epub 2018 Jul 18. Dev Neurobiol. 2018. PMID: 30022611 Free PMC article.
-
Synaptic and memory dysfunction induced by tau oligomers is rescued by up-regulation of the nitric oxide cascade.Mol Neurodegener. 2019 Jun 27;14(1):26. doi: 10.1186/s13024-019-0326-4. Mol Neurodegener. 2019. PMID: 31248451 Free PMC article.
-
The type II cGMP dependent protein kinase regulates GluA1 levels at the plasma membrane of developing cerebellar granule cells.Biochim Biophys Acta. 2013 Aug;1833(8):1820-31. doi: 10.1016/j.bbamcr.2013.03.021. Epub 2013 Mar 29. Biochim Biophys Acta. 2013. PMID: 23545413 Free PMC article.
References
-
- Alder J, Xie ZP, Valtorta F, Greengard P, Poo M. Antibodies to synaptophysin interfere with transmitter secretion at neuromuscular synapses. Neuron. 1992;9:759–768. - PubMed
-
- Antonova I, Trillat A-C, Arancio O, Zablow L, Kandel ER, Hawkins RD. Rapid increase in immunoreactivity for presynaptic proteins during long-lasting potentiation. Soc Neurosci Abstr. 2000;26:363.
-
- Arancio O, Kandel ER, Hawkins RD. Activity-dependent long-term enhancement of transmitter release by presynaptic 3′,5′-cyclic GMP in cultured hippocampal neurons. Nature. 1995;376:74–80. - PubMed
-
- Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD. Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell. 1996;87:1025–1035. - PubMed
-
- Bekkers JM, Stevens CF. Presynaptic mechanisms for long-term potentiation in the hippocampus. Nature. 1990;346:724–729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
