The external granule layer of the developing chick cerebellum generates granule cells and cells of the isthmus and rostral hindbrain
- PMID: 11150332
- PMCID: PMC6762447
- DOI: 10.1523/JNEUROSCI.21-01-00159.2001
The external granule layer of the developing chick cerebellum generates granule cells and cells of the isthmus and rostral hindbrain
Abstract
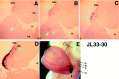
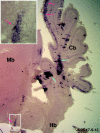
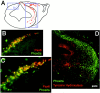
The external granule layer (EGL) on the dorsal surface of the developing cerebellum consists of neural progenitors originating from the rostral rhombic lip (RRL). The RRL and the EGL were thought to give rise exclusively to the granule neurons of the cerebellum (Alder et al., 1996). To study the fate of individual RRL cells, we used a retroviral library to mark clones in the chick embryo at Hamberger-Hamilton stages 10-12. RRL clones comprised the EGL and cerebellar granule cells, as expected. Surprisingly, however, as many as 50% of the RRL clones also contained cells ventral to the cerebellum proper. Ventral derivatives were found in clones with a medial origin, as well as in those with a lateral origin along the RRL. Some of the ventral progeny appeared to be in the process of migration, whereas others appeared to be differentiating neurons in the isthmus and the rostral hindbrain region, including the locus coeruleus (LC) and pontine reticular formation. Furthermore, the Phox2a marker of LC precursors was detected in the EGL within the anterior aspect of the cerebellum. A stream of cells originating in the EGL and expressing Phox2a was observed to terminate ventrally in the LC. These data demonstrate that single RRL progenitor cells are not restricted to producing only cerebellar granule cells; they produce both cerebellar granule cells and ventral derivatives, some of which become hindbrain neurons. They also suggest that some progeny of the EGL escape the cerebellum via the anterior aspect of the cerebellar peduncles, to contribute to the generation of ventral structures such as the LC.
Figures





Similar articles
-
Segmental identity and cerebellar granule cell induction in rhombomere 1.BMC Biol. 2004 Jun 15;2:14. doi: 10.1186/1741-7007-2-14. BMC Biol. 2004. PMID: 15198802 Free PMC article.
-
Hindbrain rhombic lip is comprised of discrete progenitor cell populations allocated by Pax6.Neuron. 2005 Dec 22;48(6):933-47. doi: 10.1016/j.neuron.2005.11.031. Neuron. 2005. PMID: 16364898
-
Cux2 serves as a novel lineage marker of granule cell layer neurons from the rhombic lip in mouse and chick embryos.Dev Dyn. 2016 Aug;245(8):881-96. doi: 10.1002/dvdy.24418. Epub 2016 Jun 13. Dev Dyn. 2016. PMID: 27198720
-
The generation of granule cells during the development and evolution of the cerebellum.Dev Dyn. 2019 Jul;248(7):506-513. doi: 10.1002/dvdy.64. Epub 2019 Jun 10. Dev Dyn. 2019. PMID: 31131952 Review.
-
Fate restriction and developmental potential of cerebellar progenitors. Transplantation studies in the developing CNS.Prog Brain Res. 2005;148:57-68. doi: 10.1016/S0079-6123(04)48006-6. Prog Brain Res. 2005. PMID: 15661181 Review.
Cited by
-
Comparative aspects of adult neural stem cell activity in vertebrates.Dev Genes Evol. 2013 Mar;223(1-2):131-47. doi: 10.1007/s00427-012-0425-5. Epub 2012 Nov 22. Dev Genes Evol. 2013. PMID: 23179636 Review.
-
The autism susceptibility gene met regulates zebrafish cerebellar development and facial motor neuron migration.Dev Biol. 2009 Nov 1;335(1):78-92. doi: 10.1016/j.ydbio.2009.08.024. Epub 2009 Sep 2. Dev Biol. 2009. PMID: 19732764 Free PMC article.
-
Lack of the central nervous system- and neural crest-expressed forkhead gene Foxs1 affects motor function and body weight.Mol Cell Biol. 2005 Jul;25(13):5616-25. doi: 10.1128/MCB.25.13.5616-5625.2005. Mol Cell Biol. 2005. PMID: 15964817 Free PMC article.
-
FGF signaling mediates regeneration of the differentiating cerebellum through repatterning of the anterior hindbrain and reinitiation of neuronal migration.J Neurosci. 2006 Jul 5;26(27):7293-304. doi: 10.1523/JNEUROSCI.0095-06.2006. J Neurosci. 2006. PMID: 16822987 Free PMC article.
-
Effect of Experimental Litter Reduction on Cerebellum Development in Suckling Rats.Bull Exp Biol Med. 2024 Oct;177(6):797-801. doi: 10.1007/s10517-024-06270-1. Epub 2024 Oct 26. Bull Exp Biol Med. 2024. PMID: 39455497
References
-
- Alcantara S, Ruiz M, De Castro, Sotelo C, Soriano E. Netrin 1 acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development. 2000;127:1359–1372. - PubMed
-
- Alder J, Cho N, Hatten ME. Embryonic precursor cells from the rhombic lip are specified to a cerebellar neuronal identity. Neuron. 1996;17:389–399. - PubMed
-
- Alder J, Lee KJ, Jessell TM, Hatten ME. Generation of cerebellar granule neurons in vivo by transplantation of BMP-treated neural progenitor cells. Nat Neurosci. 1999;2:535–540. - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes (see comments). Science. 1997;278:474–476. - PubMed
-
- Cepko CL, Golden JA, Szele FG, Lin JC. Lineage analysis in the vertebrate central nervous system. In: Cowan WM, Jessel T, Zipursky SL, editors. Molecular and cellular approaches to neural development. Oxford UP; London: 1997.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
