C/EBP beta and Elk-1 synergistically transactivate the c-fos serum response element
- PMID: 11151091
- PMCID: PMC29063
- DOI: 10.1186/1471-2121-1-2
C/EBP beta and Elk-1 synergistically transactivate the c-fos serum response element
Abstract
Background: The serum response element (SRE) in the c-fos promoter is a convergence point for several signaling pathways that regulate induction of the c-fos gene. Many transcription factors regulate the SRE, including serum response factor (SRF), ternary complex factor (TCF), and CCAAT/enhancer binding protein-beta (C/EBPbeta). Independently, the TCFs and C/EBPbeta have been shown to interact with SRF and to respond to Ras-dependent signaling pathways that result in transactivation of the SRE. Due to these common observations, we addressed the possibility that C/EBPbeta and Elk-1 could both be necessary for Ras-stimulated transactivation of the SRE.
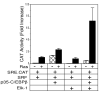
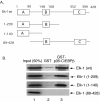
Results: In this report, we demonstrate that Elk-1 and C/EBPbeta functionally synergize in transactivation of both a Gal4 reporter plasmid in concert with Gal4-SRF and in transactivation of the SRE. Interestingly, this synergy is only observed upon activation of Ras-dependent signaling pathways. Furthermore, we show that Elk-1 and C/EBPbeta could interact both in an in vitro GST-pulldown assay and in an in vivo co-immunoprecipitation assay. The in vivo interaction between the two proteins is dependent on the presence of activated Ras. We have also shown that the C-terminal domain of C/EBPbeta and the N-terminal domain of Elk-1 are necessary for the proteins to interact.
Conclusions: These data show that C/EBPbeta and Elk-1 synergize in SRF dependent transcription of both a Gal-4 reporter and the SRE. This suggests that SRF, TCF, and C/EBPbeta are all necessary for maximal induction of the c-fos SRE in response to mitogenic signaling by Ras.
Figures




References
-
- Treisman R. The SRE: a growth factor responsive transcriptional regulator. Semin Cancer Biol. 1990;1:47–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

