Characterization of cell lines carrying self-replicating hepatitis C virus RNAs
- PMID: 11152498
- PMCID: PMC114031
- DOI: 10.1128/JVI.75.3.1252-1264.2001
Characterization of cell lines carrying self-replicating hepatitis C virus RNAs
Abstract
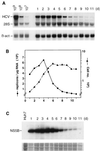
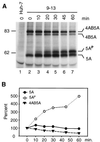
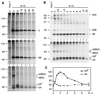
Subgenomic selectable RNAs of the hepatitis C virus (HCV) have recently been shown to self-replicate to high levels in the human hepatoma cell line Huh-7 (V. Lohmann, F. Körner, J. O. Koch, U. Herian, L. Theilmann, and R. Bartenschlager, Science 285:110-113, 1999). Taking advantage of this cell culture system that allows analyses of the interplay between HCV replication and the host cell, in this study we characterized two replicon-harboring cell lines that have been cultivated for more than 1 year. During this time, we observed no signs of cytopathogenicity such as reduction of growth rates or ultrastructural changes. High levels of HCV RNAs were preserved in cells passaged under continuous selection. When selective pressure was omitted replicon levels dropped, but depending on culture conditions the RNAs persisted for more than 10 months. A tight coupling of the amounts of HCV RNA and proteins to host cell growth was observed. Highest levels were found in exponentially growing cells, followed by a sharp decline in resting cells, suggesting that cellular factors required for RNA replication and/or translation vary in abundance and become limiting in resting cells. Studies of polyprotein processing revealed rapid cleavages at the NS3/4A and NS5A/B sites resulting in a rather stable NS4AB5A precursor that was processed slowly into individual products. Half-lives (t(1/2)s) of mature proteins ranged from 10 to 16 h, with the exception of the hyperphosphorylated form of NS5A, which was less stable (t(1/2), approximately 7 h). Results of immunoelectron microscopy revealed an association of the majority of viral proteins with membranes of the endoplasmic reticulum, suggesting that this is the site of RNA replication. In summary, replicon-bearing cells are a good model for viral persistence, and they allow the study of various aspects of the HCV life cycle.
Figures







References
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1987.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

