Identification of novel HLA-A2-restricted human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte epitopes predicted by the HLA-A2 supertype peptide-binding motif
- PMID: 11152503
- PMCID: PMC114036
- DOI: 10.1128/JVI.75.3.1301-1311.2001
Identification of novel HLA-A2-restricted human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte epitopes predicted by the HLA-A2 supertype peptide-binding motif
Abstract
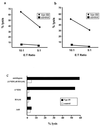
Virus-specific cytotoxic T-lymphocyte (CTL) responses are critical in the control of human immunodeficiency virus type 1 (HIV-1) infection and will play an important part in therapeutic and prophylactic HIV-1 vaccines. The identification of virus-specific epitopes that are efficiently recognized by CTL is the first step in the development of future vaccines. Here we describe the immunological characterization of a number of novel HIV-1-specific, HLA-A2-restricted CTL epitopes that share a high degree of conservation within HIV-1 and a strong binding to different alleles of the HLA-A2 superfamily. These novel epitopes include the first reported CTL epitope in the Vpr protein. Two of the novel epitopes were immunodominant among the HLA-A2-restricted CTL responses of individuals with acute and chronic HIV-1 infection. The novel CTL epitopes identified here should be included in future vaccines designed to induce HIV-1-specific CTL responses restricted by the HLA-A2 superfamily and will be important to assess in immunogenicity studies in infected persons and in uninfected recipients of candidate HIV-1 vaccines.
Figures


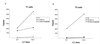
References
-
- Allen T M, O'Connor D H, Jing P, Dzuris J L, Mothe B R, Vogel T U, Dunphy E, Liebl M E, Emerson C, Wilson N, Kunstman K J, Wang X, Allison D B, Hughes A L, Desrosiers R C, Altman J D, Wolinsky S M, Sette A, Watkins D I. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. 2000;407:386–390. - PubMed
-
- Altfeld M, Rosenberg E S. The role of CD4(+) T helper cells in the cytotoxic T lymphocyte response to HIV-1. Curr Opin Immunol. 2000;12:375–380. - PubMed
-
- Altfeld, M. A., E. S. Rosenberg, R. Shankarappa, J. S. Mukherjee, R. Hecht, R. L. Eldridge, M. M. Addo, S. H. Poon, M. N. Phillips, G. Robbins, J. O. Kahn, C. Brander, P. J. Goulder, J. A. Levy, J. I. Mullins, and B. D. Walker. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med., in press. - PMC - PubMed
-
- Altfeld M A, Trocha A, Eldridge R L, Rosenberg E S, Phillips M N, Addo M M, Sekaly R P, Kalams S A, Burchett S A, McIntosh K, Walker B D, Goulder P J. Identification of dominant optimal HLA-B60- and HLA-B61-restricted cytotoxic T-lymphocyte (CTL) epitopes: rapid characterization of CTL responses by enzyme-linked immunospot assay. J Virol. 2000;74:8541–8549. - PMC - PubMed
-
- Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

