Activation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus
- PMID: 11152511
- PMCID: PMC114044
- DOI: 10.1128/JVI.75.3.1378-1386.2001
Activation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus
Abstract
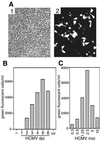
The majority of Kaposi's sarcoma-associated herpesvirus (KSHV)-infected cells identified in vivo contain latent KSHV, with lytic replication in only a few percent of cells, as is the case for the cells of Kaposi's sarcoma (KS) lesions. Factors that influence KSHV latent or lytic replication are not well defined. Because persons with KS are often immunosuppressed and susceptible to many infectious agents, including human cytomegalovirus (HCMV), we have investigated the potential for HCMV to influence the replication of KSHV. Important to this work was the construction of a recombinant KSHV, rKSHV.152, expressing the green fluorescent protein (GFP) and neo (conferring resistance to G418). The expression of GFP was a marker of KSHV infection in cells of both epithelial and endothelial origin. The rKSHV.152 virus was used to establish cells, including human fibroblasts (HF), containing only latent KSHV, as demonstrated by latency-associated nuclear antigen expression and Gardella gel analysis. HCMV infection of KSHV latently infected HF activated KSHV lytic replication with the production of infectious KSHV. Dual-color immunofluorescence detected both the KSHV lytic open reading frame 59 protein and the HCMV glycoprotein B in coinfected cells, and UV-inactivated HCMV did not activate the production of infectious KSHV-GFP. In addition, HCMV coinfection increased the production of KSHV from endothelial cells and activated lytic cycle gene expression in keratinocytes. These data demonstrate that HCMV can activate KSHV lytic replication and suggest that HCMV could influence KSHV pathogenesis.
Figures
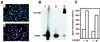
Similar articles
-
Use of the red fluorescent protein as a marker of Kaposi's sarcoma-associated herpesvirus lytic gene expression.Virology. 2004 Aug 1;325(2):225-40. doi: 10.1016/j.virol.2004.03.049. Virology. 2004. PMID: 15246263
-
Activation of Kaposi's sarcoma-associated herpesvirus lytic gene expression during epithelial differentiation.J Virol. 2005 Nov;79(21):13769-77. doi: 10.1128/JVI.79.21.13769-13777.2005. J Virol. 2005. PMID: 16227296 Free PMC article.
-
Glycolysis, Glutaminolysis, and Fatty Acid Synthesis Are Required for Distinct Stages of Kaposi's Sarcoma-Associated Herpesvirus Lytic Replication.J Virol. 2017 Apr 28;91(10):e02237-16. doi: 10.1128/JVI.02237-16. Print 2017 May 15. J Virol. 2017. PMID: 28275189 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus: the role of lytic replication in targeted therapy.Curr Opin Infect Dis. 2015 Dec;28(6):611-24. doi: 10.1097/QCO.0000000000000213. Curr Opin Infect Dis. 2015. PMID: 26524334 Review.
-
The chromatin landscape of Kaposi's sarcoma-associated herpesvirus.Viruses. 2013 May 23;5(5):1346-73. doi: 10.3390/v5051346. Viruses. 2013. PMID: 23698402 Free PMC article. Review.
Cited by
-
Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis.J Virol. 2002 Jun;76(12):6185-96. doi: 10.1128/jvi.76.12.6185-6196.2002. J Virol. 2002. PMID: 12021352 Free PMC article.
-
Correlation between infections with different genotypes of human cytomegalovirus and Epstein-Barr virus in subgingival samples and periodontal status of patients.J Clin Microbiol. 2007 Nov;45(11):3665-70. doi: 10.1128/JCM.00374-07. Epub 2007 Sep 5. J Clin Microbiol. 2007. PMID: 17804655 Free PMC article.
-
Lipid rafts of primary endothelial cells are essential for Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry.J Virol. 2007 Aug;81(15):7941-59. doi: 10.1128/JVI.02848-06. Epub 2007 May 16. J Virol. 2007. PMID: 17507466 Free PMC article.
-
Evidence of inability of human cytomegalovirus to reactivate Kaposi's sarcoma-associated herpesvirus from latency in body cavity-based lymphocytes.J Clin Virol. 2009 Nov;46(3):244-8. doi: 10.1016/j.jcv.2009.07.025. Epub 2009 Sep 1. J Clin Virol. 2009. PMID: 19726225 Free PMC article.
-
Infection of primary human tonsillar lymphoid cells by KSHV reveals frequent but abortive infection of T cells.Virology. 2011 Apr 25;413(1):1-11. doi: 10.1016/j.virol.2010.12.036. Epub 2011 Feb 25. Virology. 2011. PMID: 21353276 Free PMC article.
References
-
- Adams V, Kempf W, Hassam S, Briner J, Schmid M, Moos R, Pfaltz M. Detection of several types of human papilloma viruses in AIDS-associated Kaposi's sarcoma. J Med Virol. 1995;46:189–193. - PubMed
-
- Ambinder R F, Robertson K D, Tao Q. DNA methylation and the Epstein-Barr virus. Semin Cancer Biol. 1999;9:369–375. - PubMed
-
- Beral V, Newton R. Overview of the epidemiology of immunodeficiency-associated cancers. J Natl Cancer Inst Monogr. 1998;23:1–6. - PubMed
-
- Beral V, Peterman T A, Berkelman R L, Jaffe H W. Kaposi's sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123–128. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

