Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein
- PMID: 11152512
- PMCID: PMC114045
- DOI: 10.1128/JVI.75.3.1387-1400.2001
Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein
Abstract
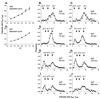
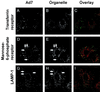
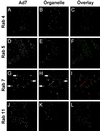
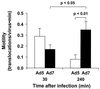
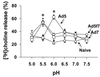
The intracellular trafficking of adenovirus (Ad) subgroup B (e.g., Ad7) differs from that of subgroup C (e.g., Ad5) in that Ad5 rapidly escapes from endocytic compartments following infection whereas Ad7 accumulates in organelles. To assess the hypothesis that Ad7 is targeted to the lysosomal pathway, Ad7 and Ad5 were conjugated with fluorophores and their trafficking in A549 epithelial cells was analyzed by fluorescence microscopy. Within 1 h after infection, Ad7, but not Ad5, accumulated in the cytoplasm of A549 cells. The pH in the environment of Ad5 was nearly neutral (pH 7), while Ad7 occupied acidic compartments (pH 5) over the first 2 h with a gradual shift toward neutrality by 8 h. Ad7 partially colocalized with alpha(2)-macroglobulin and late endosomal and lysosomal marker proteins, including Rab7, mannose-6-phosphate receptor, and LAMP-1. The pH optimum for membrane lysis by Ad7, as well as a chimeric Ad5 capsid that expressed the Ad7 fiber (Ad5fiber7), was pH 5.5, while that for lysis by Ad5 was pH 6.0. Thus, the native trafficking pathway for Ad7 involves residence in late endosomes and lysosomes, with information encoded in the Ad7 fiber acting as a pH-dependent trigger for membrane lysis and escape to the cytosol.
Figures





Similar articles
-
The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses.J Virol. 2003 Mar;77(6):3712-23. doi: 10.1128/jvi.77.6.3712-3723.2003. J Virol. 2003. PMID: 12610146 Free PMC article.
-
Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors.J Virol. 1999 Jul;73(7):6056-65. doi: 10.1128/JVI.73.7.6056-6065.1999. J Virol. 1999. PMID: 10364358 Free PMC article.
-
Role for the adenovirus IVa2 protein in packaging of viral DNA.J Virol. 2001 Nov;75(21):10446-54. doi: 10.1128/JVI.75.21.10446-10454.2001. J Virol. 2001. PMID: 11581412 Free PMC article.
-
[Progress in infection pathway and intracellular trafficking of adenovirus].Sheng Wu Gong Cheng Xue Bao. 2014 Jun;30(6):864-74. Sheng Wu Gong Cheng Xue Bao. 2014. PMID: 25212004 Review. Chinese.
-
Intracellular trafficking of adenovirus: many means to many ends.Adv Drug Deliv Rev. 2007 Aug 10;59(8):810-21. doi: 10.1016/j.addr.2007.06.007. Epub 2007 Jun 28. Adv Drug Deliv Rev. 2007. PMID: 17707546 Review.
Cited by
-
Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys.J Virol. 2012 Sep;86(18):9590-8. doi: 10.1128/JVI.00740-12. Epub 2012 Jul 11. J Virol. 2012. PMID: 22787208 Free PMC article.
-
Microtubule-mediated and microtubule-independent transport of adenovirus type 5 in HEK293 cells.J Virol. 2007 Jul;81(13):6899-908. doi: 10.1128/JVI.02330-05. Epub 2007 Apr 18. J Virol. 2007. PMID: 17442712 Free PMC article.
-
The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses.J Virol. 2003 Mar;77(6):3712-23. doi: 10.1128/jvi.77.6.3712-3723.2003. J Virol. 2003. PMID: 12610146 Free PMC article.
-
Adenovirus receptors.J Virol. 2005 Oct;79(19):12125-31. doi: 10.1128/JVI.79.19.12125-12131.2005. J Virol. 2005. PMID: 16160140 Free PMC article. Review. No abstract available.
-
Opening of size-selective pores in endosomes during human rhinovirus serotype 2 in vivo uncoating monitored by single-organelle flow analysis.J Virol. 2005 Jan;79(2):1008-16. doi: 10.1128/JVI.79.2.1008-1016.2005. J Virol. 2005. PMID: 15613329 Free PMC article.
References
-
- Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. - PubMed
-
- Blumenthal R, Seth P, Willingham M C, Pastan I. pH-dependent lysis of liposomes by adenovirus. Biochemistry. 1986;25:2231–2237. - PubMed
-
- Chardonnet Y, Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970a;40:462–477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

