Genetic and biochemical analysis of the yeast plasma membrane Ssy1p-Ptr3p-Ssy5p sensor of extracellular amino acids
- PMID: 11154269
- PMCID: PMC86673
- DOI: 10.1128/MCB.21.3.814-826.2001
Genetic and biochemical analysis of the yeast plasma membrane Ssy1p-Ptr3p-Ssy5p sensor of extracellular amino acids
Abstract
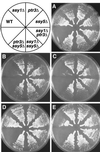
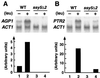
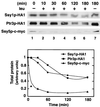
Ssy1p and Ptr3p are known components of a yeast plasma membrane system that functions to sense the presence of amino acids in the extracellular environment. In response to amino acids, this sensing system initiates metabolic signals that ultimately regulate the functional expression of several amino acid-metabolizing enzymes and transport proteins, including multiple, genetically distinct amino acid permeases. We have found that SSY5 encodes a third component of this amino acid sensing system. Mutations in SSY5 manifest phenotypes that are indistinguishable from those resulting from either single ssy1 and ptr3 mutations or ssy5 ssy1 and ssy5 ptr3 double mutations. Although Ssy5p is predicted to be a soluble protein, it exhibits properties indicating that it is a peripherally associated plasma membrane protein. Each of the three sensor components, Ssy1p, Ptr3p, and Ssy5p, adopts conformations and modifications that are dependent upon the availability of amino acids and on the presence of the other two components. These results suggest that these components function as part of a sensor complex localized to the plasma membrane. Consistent with a sensor complex, the overexpression of SSY1 or the unique N-terminal extension of this amino acid permease homologue inactivates the amino acid sensor in a dominant-negative manner. Each of the components of the Ssy1p-Ptr3p-Ssy5p (SPS) signaling system undergoes rapid physical changes, reflected in altered electrophoretic mobility, when leucine is added to cells grown in media lacking amino acids. Furthermore, the levels of each SPS sensor component present in whole-cell extracts diminish upon leucine addition. The rapid physical alterations and reduced levels of sensor components are consistent with their being downregulated in response to amino acid availability. These results reveal the dynamic nature of the amino acid-initiated signals transduced by the SPS sensor.
Figures






Similar articles
-
Amino acid signaling in yeast: casein kinase I and the Ssy5 endoprotease are key determinants of endoproteolytic activation of the membrane-bound Stp1 transcription factor.Mol Cell Biol. 2004 Nov;24(22):9771-85. doi: 10.1128/MCB.24.22.9771-9785.2004. Mol Cell Biol. 2004. PMID: 15509782 Free PMC article.
-
Hyper- and hyporesponsive mutant forms of the Saccharomyces cerevisiae Ssy1 amino acid sensor.Mol Membr Biol. 2008 Feb;25(2):164-76. doi: 10.1080/09687680701771917. Mol Membr Biol. 2008. PMID: 18307103
-
Ssy1p and Ptr3p are plasma membrane components of a yeast system that senses extracellular amino acids.Mol Cell Biol. 1999 Aug;19(8):5405-16. doi: 10.1128/MCB.19.8.5405. Mol Cell Biol. 1999. PMID: 10409731 Free PMC article.
-
Amino-acid-induced signalling via the SPS-sensing pathway in yeast.Biochem Soc Trans. 2009 Feb;37(Pt 1):242-7. doi: 10.1042/BST0370242. Biochem Soc Trans. 2009. PMID: 19143640 Review.
-
Research progress on the function and regulatory pathways of amino acid permeases in fungi.World J Microbiol Biotechnol. 2024 Nov 25;40(12):392. doi: 10.1007/s11274-024-04199-1. World J Microbiol Biotechnol. 2024. PMID: 39581943 Review.
Cited by
-
Suppressors of ssy1 and ptr3 null mutations define novel amino acid sensor-independent genes in Saccharomyces cerevisiae.Genetics. 2001 Jul;158(3):973-88. doi: 10.1093/genetics/158.3.973. Genetics. 2001. PMID: 11454748 Free PMC article.
-
A phosphodegron controls nutrient-induced proteasomal activation of the signaling protease Ssy5.Mol Biol Cell. 2011 Aug 1;22(15):2754-65. doi: 10.1091/mbc.E11-04-0282. Epub 2011 Jun 8. Mol Biol Cell. 2011. PMID: 21653827 Free PMC article.
-
Differential regulation of transcription factors Stp1 and Stp2 in the Ssy1-Ptr3-Ssy5 amino acid sensing pathway.J Biol Chem. 2011 Feb 11;286(6):4620-31. doi: 10.1074/jbc.M110.195313. Epub 2010 Dec 2. J Biol Chem. 2011. PMID: 21127045 Free PMC article.
-
Regulation of nutrient transporters by metabolic and environmental stresses.Curr Opin Cell Biol. 2020 Aug;65:35-41. doi: 10.1016/j.ceb.2020.02.009. Epub 2020 Mar 19. Curr Opin Cell Biol. 2020. PMID: 32200208 Free PMC article. Review.
-
AgtA, the dicarboxylic amino acid transporter of Aspergillus nidulans, is concertedly down-regulated by exquisite sensitivity to nitrogen metabolite repression and ammonium-elicited endocytosis.Eukaryot Cell. 2009 Mar;8(3):339-52. doi: 10.1128/EC.00270-08. Epub 2009 Jan 23. Eukaryot Cell. 2009. PMID: 19168757 Free PMC article.
References
-
- Allen J B, Elledge S J. A family of vectors that facilitate transposon and insertional mutagenesis of cloned genes in yeast. Yeast. 1994;10:1267–1272. - PubMed
-
- Aronheim A, Engelberg D, Li N, al-Alawi N, Schlessinger J, Karin M. Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell. 1994;78:949–961. - PubMed
-
- Barnes D, Lai W, Breslav M, Naider F, Becker J M. PTR3, a novel gene mediating amino acid-inducible regulation of peptide transport in Saccharomyces cerevisiae. Mol Microbiol. 1998;29:297–310. - PubMed
-
- Barral Y, Mann C. G1 cyclin degradation and cell differentiation in Saccharomyces cerevisiae. C R Acad Sci Sec III Life Sci. 1995;318:43–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
